A car battery is actually a series of cells. A single electric cell can be made by
Question:
A car battery is actually a series of cells. A single electric cell can be made by placing two plates of different materials that have different affinities for electrons in a conducting solution. You can make a simple 1.5-V cell by placing a strip of copper and a strip of zinc in a tumbler of salt water. The voltage of a cell depends on the materials used and on the conducting solution they are placed in, not on the size of the plates.
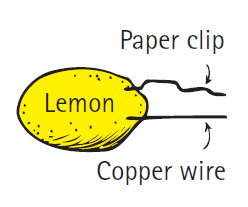
An easier cell to construct is the citrus cell. Stick a paper clip and a piece of copper wire into a lemon. Hold the ends of the wire close together, but not touching, and place the ends on your tongue. The slight tingle you feel and the metallic taste you experience result from a small current of electricity pushed by the citrus cell through the wires when your moist tongue closes the circuit.
Step by Step Answer:

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt





