Question: According to Figure 19.3, which has the higher boiling point: gasoline or kerosene? Figure 19.3 - Natural gas COOLER Gasoline Fractionating tower Kerosene Pipe still
According to Figure 19.3, which has the higher boiling point: gasoline or kerosene?
Figure 19.3
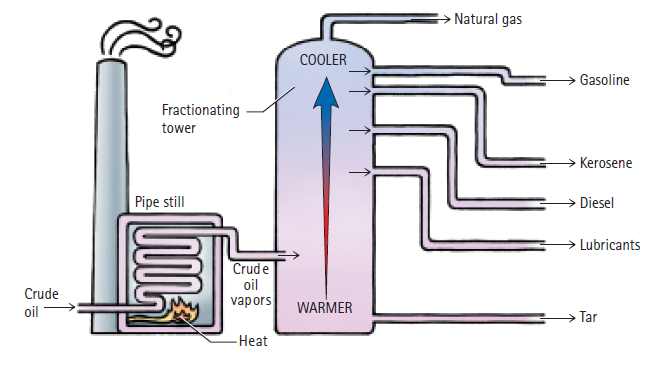
- Natural gas COOLER Gasoline Fractionating tower Kerosene Pipe still Diesel Lubricants Crud e oil Crude vapors WARMER oil Tar -eat
Step by Step Solution
3.32 Rating (161 Votes )
There are 3 Steps involved in it
To make it to the top of the fractionating column a substance must re... View full answer

Get step-by-step solutions from verified subject matter experts


