Estimate whether entropy increases or deceases with the following reaction. Use data from Table 17.1 to confirm
Question:
Estimate whether entropy increases or deceases with the following reaction. Use data from Table 17.1 to confirm your estimate.
2 C(s) + 3 H2(g) → C2H6(g)
Table 17.1
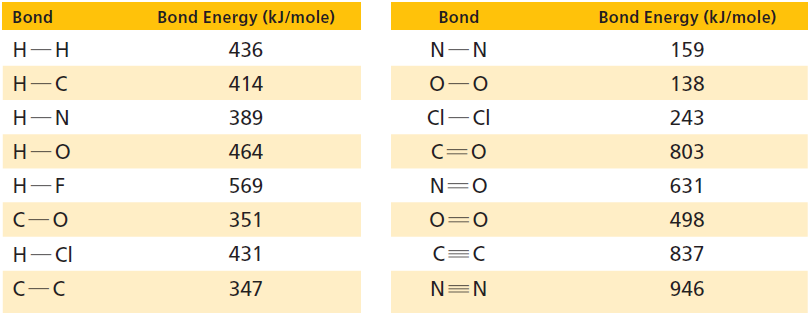
Transcribed Image Text:
Bond Energy (kJ/mole) Bond Energy (kJ/mole) Bond Bond Н-Н 436 159 Н-С 414 138 Cl-CI 389 Н—N 243 803 Н—О 464 C=0 Н-—F 569 N=0 631 351 498 Н— СІ 431 837 N=N 347 946
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (9 reviews)
Consider how this reaction shows the formation of a single molec...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:
Students also viewed these Physics questions
-
Estimation of diffusivity for a binary mixture at high density, Predict CD AB for an equimolar mixture of N 2 and C 2 H 6 at 288.2K and 40atm (a) Use the value of D AB at 1atm from Table 17.1-1,...
-
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride...
-
How many grams of gallium are there in a 145-gram sample of gallium arenside, GaAs?
-
Find the magnitude of the reaction at A and the tension in cable CD using (a) the con- cepts of three-force bodies (closed polygon method discussed in chapter 2). (b) Check your answers by doing sum...
-
Kane Legal Services provides legal advice to clients. The following data apply to the first six months of operation: Required a. What is the average service revenue per hour for the six-month time...
-
Brad and Valerie decided to adopt a child and contacted an adoption agency in August 2015. After extensive interviews and other requirements (such as financial status, etc.), Brad and Valerie were...
-
Appreciate the changes taking place in the fashion system. LO.1
-
a. Freds Hardware and Hobby House expects its sales to increase at a constant rate of 8 percent per year over the next three years. Current sales are $100,000. Forecast sales for each of the next...
-
How does a financial institution differ from an industrial corporation in terms of the management of risk?
-
Every time a machine breaks down at the Dynaco Manufacturing Company (Problem 3), either 1, 2, or 3 hours are required to fix it, according to the following probability distribution: Repair Time...
-
According to the second law of thermodynamics, exothermic reactions, such as the burning of wood, are favored because they result in the dispersal of energy. Wood, however, will not spontaneously...
-
Exothermic reactions are favored because they release heat to the environment. Would an exothermic reaction be more favored or less favored if it were carried out within a superheated chamber?
-
a. Use a graphing utility to graph y = 2x 2 - 82x + 720 in a standard viewing rectangle. What do you observe? b. Find the coordinates of the vertex for the given quadratic function. c. The answer to...
-
How do I record these entries? January 1: Purchased a fleet of vehicles for $350,000 via a loan from the bank. The trucks have a useful life of six years. The loan is for six years with an interest...
-
How do feedback mechanisms and performance evaluation systems contribute to individual and team development within a corporate context ?
-
How do ideological frameworks underpin political movements, and what is their role in legitimizing or challenging power structures?
-
According to the 8-step communication model, what should one do once the crisis has passed?
-
Who is PGR and what do they do? How much premium did the company (consolidated) write in the most recent complete fiscal year? Was it a good, average, or bad year for them? Comment on the premium...
-
Find all values of x in the interval [0, 2] that satisfy the equation. 2 cos x + sin 2x = 0
-
Pearson Education, a publisher of college textbooks, would like to know if students prefer traditional textbooks or digital textbooks. A random sample of students was asked their preference and the...
-
Consider the circuit shown in Fig. P25.73. The emf source has negligible internal resistance. The resistors have resistances R 1 = 6.00 Ω and R 2 = 4.00 Ω. The capacitor has...
-
Consider the circuit shown in Fig. P25.72. The battery has emf 72.0 V and negligible internal resistance. R 2 = 2.00 Ω, C 1 = 3.00 µF, and C 2 = 6.00 µF. After the capacitors...
-
Compact fluorescent bulbs are much more efficient at producing light than are ordinary incandescent bulbs. They initially cost much more, but they last far longer and use much less electricity....
-
The payroll register of Ruggerio Co. indicates $13,800 of social security withheld and $3,450 of Medicare tax withheld on total salaries of $230,000 for the period. Federal withholding for the period...
-
All of the following are included on Form 1040, page 1, EXCEPT: The determination of filing status. The Presidential Election Campaign check box. The income section. The paid preparer signature line.
-
Question One: (25 marks) (X) Inc. purchased 80% of the outstanding voting shares of (Y) for $360,000 on July 1, 2017. On that date, (Y) had common shares and retained earnings worth $180,000 and...

Study smarter with the SolutionInn App


