Rank the following molecules in order of the phase they form at room temperature: solid, liquid, gas.
Question:
Rank the following molecules in order of the phase they form at room temperature: solid, liquid, gas.
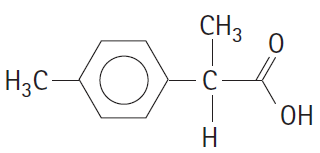
a.
b. CH3CH2CH2CH3
c. CH3CH2CH2CH2 - OH
Transcribed Image Text:
CHз H;C- C- ОН Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
a So...View the full answer

Answered By

Ayush Mishra
I am a certified online tutor, with more than 3 years of experience in online tutoring. My tutoring subjects include: Physics, Mathematics and Mechanical engineering. I have also been awarded as best tutor for year 2019 in my previous organisation. Being a Mechanical Engineer, I love to tell the application of the concepts of science and mathematics in the real world. This help students to develop interest and makes learning fun and easy. This in turn, automatically improves their grades in the subject. I teach students to get prepared for college entry level exam. I also use to teach undergraduate students and guide them through their career aim.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:
Students also viewed these Physics questions
-
Rank the following molecules in order of increasing boiling point (without looking up the real values!): (a) 3-methylheptane; (b) Octane; (c) 2,4-dimethylhexane; (d) 2,2,4-trimethylpentane.
-
Rank the following hydrocarbons in order of increasing number of hydrogen atoms: (a) (b) (c)
-
Draw all the structural isomers for hydrocarbons having the molecular formula C 6 H 14 .
-
If the individual subsidiary ledger accounts contained the following data: Cadence Company, Vendor, $200, credit balance Franklin Enterprises, Customer , $750, debit balance Marcelo Construction,...
-
On June 1, 2010, Skylark Enterprises (not a corporation) acquired a retail store building for $500,000 (with $100,000 being allocated to the land). The store building was 39-year real property, and...
-
Determine the shear stress at point B on the web of the cantilevered strut at section aa. 4 kN 2 kN 300 mm 250 mm a 250 mm 20 mm 70 mm 20 mm 50 mm
-
3. Interest expense on Westminster bonds appears in the consolidated income for 2017 at: a $34,000 b $39,000 c $30,000 d None of the above
-
The data identified below was listed in a projects latest status report: BCWS = $36,000 BCWP = $30,000 ACWP = $33,000 BAC = $120,000 Original length of the project 10 months Using these data,...
-
1. What is the difference between absorption costing and variable costing? 2. Distinguish between product costs and period costs. 6. What is the difference between gross margin and manufacturing...
-
Your sister has just won $300,000 (taxfree) in the state lottery. Shes decided to quit her job and devote herself to writing novels for the next ten years, using her lottery winnings to support...
-
A property of polymers is their glass transition temperature, T g , which is the approximate temperature below which the polymer is hard and rigid, but above which the polymer is soft and flexible....
-
Rank the following organic molecules in order of increasing solubility in water: (b) (c) (a)
-
Explain the advantages of cost audit.
-
Arizona Corp. had the following account balances at 12/1/19: Receivables: $96,000; Inventory: $240,000; Land: $720,000; Building: $600,000; Liabilities: $480,000; Common stock: $120,000; Additional...
-
Construct a 90% confidence interval for the population standard deviation o at Bank A. Bank A 4.2 5.4 5.9 6.1 6.6 7.7 7.7 8.6 9.3 10.0
-
Margin of Error For the poll described in Exercise 1, describe what is meant by the statement that "the margin of error was given as +3.5 percentage points."
-
1) Explain what the critical issue was in the Uber decisions in the First Circuit (Culliane case), and the Second Circuit (Myer case), and how each court, looking at the same facts, came to opposite...
-
IFRS LEASE 1. Kappa Berhad enters into a 10-year lease on 1 January 2020. Kappa Berhad incurred the following costs in respect of the lease: RM2,500 legal fees RM15,000 deposit made at the...
-
Moonquakes are weaker and occur much less often than earthquakes. What do these facts imply about temperatures in the moons interior?
-
When the Department of Homeland Security created a color-coded system to prepare government officials and the public against terrorist attacks, what did it do right and what did it do wrong?
-
Glycerin flows parallel to a flat plate measuring 2 ft by 2 ft with a velocity of 10 fps. Determine values for the mean convective heat-transfer coefficient and the associated drag force imposed on...
-
Given the conditions specified in Problem 19.8, construct a plot of local heat-transfer coefficient vs. position along the plate for glycerin temperatures of 30F, 50F, and 80F. Data From Problem 19.8...
-
Nitrogen at 100F and 1 atm flows at a velocity of 100 fps. A flat plate 6 in. wide, at a temperature of 200F, is aligned parallel to the direction of flow. At a position 4 ft from the leading edge,...
-
Callaho Inc. began operations on January 1 , 2 0 1 8 . Its adjusted trial balance at December 3 1 , 2 0 1 9 and 2 0 2 0 is shown below. Other information regarding Callaho Inc. and its activities...
-
Required: 1. Complete the following: a. Colnpute the unit product cost under absorption costing. b. What is the company's absorption costing net operating income (loss) for the quarter? c. Reconcile...
-
Bond Valuation with Semiannual Payments Renfro Rentals has issued bonds that have an 8% coupon rate, payable semiannually. The bonds mature in 6 years, have a face value of $1,000, and a yield to...

Study smarter with the SolutionInn App


