Question: The amphoteric reaction between two water molecules is endothermic, which means the reaction requires the input of heat energy in order to proceed: Thewarmer the
The amphoteric reaction between two water molecules is endothermic, which means the reaction requires the input of heat energy in order to proceed: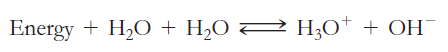 Thewarmer the water, the more heat energy is available for this reaction, and the more hydronium and hydroxide ions are formed.
Thewarmer the water, the more heat energy is available for this reaction, and the more hydronium and hydroxide ions are formed.
(a) Which has a lower pH: pure water that is hot or pure water that is cold?
(b) Is it possible for water to be neutral but to have a pH less than or greater than 7.0?
Energy + H,0 + H2O 2 H3;0* + OH
Step by Step Solution
3.31 Rating (163 Votes )
There are 3 Steps involved in it
a pH is a measure of the hydronium ion concentration The greater the hydronium ion concentration the ... View full answer

Get step-by-step solutions from verified subject matter experts


