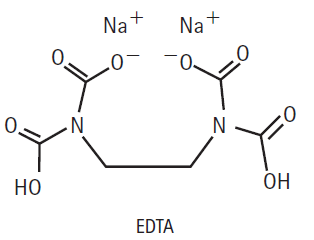
The disodium salt of ethylenediaminetetraacetic acid, also known as EDTA, has a great affinity for lead ions,
Question:
The disodium salt of ethylenediaminetetraacetic acid, also known as EDTA, has a great affinity for lead ions, Pb2+. Why? Can you think of any useful applications of this chemistry?
Transcribed Image Text:
Na+ Na + _0° N - Но ОН EDTA
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
EDTA is used to help ...View the full answer

Answered By

David Muchemi
I am a professional academic writer with considerable experience in writing business and economic related papers. I have been writing for my clients who reach out to me personally after being recommended to me by satisfied clients.
I have the English language prowess, no grammatical and spelling errors can be found in my work. I double-check for such mistakes before submitting my papers.
I deliver finished work within the stipulated time and without fail. I am a good researcher on any topic especially those perceived to be tough.
I am ready to work on your papers and ensure you receive the highest quality you are looking for. Please hire me to offer my readily available quality service.
Best regards,
4.60+
27+ Reviews
61+ Question Solved
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:
Students also viewed these Physics questions
-
Ethylenediaminetetraacetic acid displaces bismuth( III) from its thiourea complex: Bi(tu)63+ + H2Y2- ( BiY- + 6tu + 2H+ Where tu is the thiourea molecule, (NH2)2CS. Predict the shape of a photometric...
-
Epoxy adhesives are prepared in two steps. SN2 reaction of the disodium salt of bisphenol A with epichiorohydrin forms a ?prepolymer,? which is then ?cured? by treatment with a triamine such as H 2...
-
Epoxy adhesives arc cross-linked resins prepared in two steps. The first step involves S N 2 reaction of the disodium salt of bisphenol A with epichiorohydrin to form a low-molecular-weight...
-
A manufacturer of cases for sound equipment requires that holes be drilled for metal screws. The drill bits wear out and must be replaced; there is expense not only in the cost of the bits but also...
-
Consuela was a tenant in a campus apartment. She is a student at State University. Her lease began on August 1, 2014, and was due to expire on July 31, 2015. However, her landlord sold the building,...
-
The beveled gear is subjected to the loads shown. Determine the stress components acting on the shaft at point B, and show the results on a volume element located at this point. The shaft has a...
-
P7-3 Subsidiary purchases parent bonds The separate trial balance for Thanos SA and Merry SA, its 90 percent-owned subsidiary, for the year ended 2014 is as follows: Debits Thanos SA Merry SA Cash...
-
The financial statements of Coca-Cola and PepsiCo are presented in Appendices C and D, respectively. The companies complete annual reports, including the notes to the financial statements, are...
-
TJ invests in a closed end fund, buying when the NAV is $85 but shares are trading at a 3% premium. He holds onto it until the $3 income payment, when he sells at a 3% discount from the NAV, which is...
-
Which type of application benefits the most by bypassing write cache? Justify your answer. Discuss.
-
What products are formed upon the reaction of benzoic acid with sodium hydroxide, NaOH? One of these products is a common food preservative. Can you name it?
-
Would you expect polypropylene to be more dense or less dense than low-density polyethylene? Why?
-
Should organizations spend more resources monitoring their least experienced and youngest workers if they are the biggest cybersecurity risk?
-
Most research indicates that good leaders exhibit these leadership skills / https://emeritus.org/blog/leadership-skills-for-managers/ Which of these skills, in your opinion, are the most difficult to...
-
Consider the following account balances (in thousands) for the Shaker Corporation In the Dec 31.2021 Cash $200,000 and Capital $2,000,000 and Retained earnings $1,500,000 The balances of raw...
-
Given: a = -7,b=-519, c = < 5,-1,9 >,d= 2j - 4k, e = < 4, -6, -3> F = 6 -[312].G=124 -91 2x1 Determine the following if possible and if not possible explain why not. i. a ii. |c| iii. |F| iv. V. F-1...
-
I have been identified and approached by leaders who saw my potential and asked me to apply for a position. I was humbled and honored to be identified and I accepted the invitation. It has led to...
-
the object is 2.0mm?there are two converging lens on the right side of the object?one is 9.9cm far away from the object and has a focal point 9.0cm?the other is 101.1cm far away from the first lens...
-
Why is the sun considered to be a star?
-
How will relating product contribution margin s to the amount of the constrained resource they consume help a company maximize its profits?
-
For the heater consisting of the tube bank described in Problem 20.32, evaluate the heat transferred to the water if the tube array consists of six rows of tubes in the flow direction with eight...
-
A tube bank employs an in-line arrangement with S T = S L = 3.2 cm and tubes that are 1.8 cm in outside diameter. There are 10 rows of tubes, which are held at a surface temperature of 85C. Air at...
-
Rework Problem 20.51 for a staggered arrangement. Data From Problem 20.51 Cooling water flows through thin-walled tubes in a condenser with a velocity of 1.5 m/s. The tubes are 25.4 mm in diameter....
-
Industry Current Year Minus 1 Current Year Minus 2 Company: Air Products and Chemicals, Inc. (APD) Stock Price: 306.72 USD Shares Outstanding: 220.89 M Financial Ratios Most Current Year Current...
-
Assume the market portfolio of common stocks earned 14.1 percent in one year while U.S. Treasury bills earned 4.4 percent and inflation averaged 4.6 percent. What was the market risk premium?
-
A bank quotes you an interest rate of 9 . 5 % per annum with quarterly compounding. What is the equivalent rate with continuous compounding?

Study smarter with the SolutionInn App


