Which of the following diagrams best represents the size of the atomic nucleus relative to the size
Question:
Which of the following diagrams best represents the size of the atomic nucleus relative to the size of the atom?
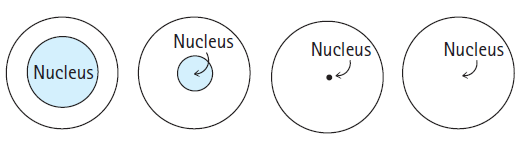
Transcribed Image Text:
Nucleus Nucleus Nucleus Nucleus)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (22 reviews)
The one on ...View the full answer

Answered By

MICHAEL KICHE
I was employed studypool for the first time in tutoring. I did well since most of my students and clients got the necessary information and knowledge requested for. I always submitted the answers in time and followed the correct formatting in answering eg MLA or APA format,
Again I worked with the writers bay where I did writing and got many clients whom we worked with so closely. They enjoyed every single service I delivered to them. My answers are always correct.
4.70+
13+ Reviews
54+ Question Solved
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:
Students also viewed these Physics questions
-
Which of the following diagrams best represents an aqueous solution of NaF? The water molecules are not shown for clarity. Will this solution be acidic, neutral, or basic?
-
Which of the following diagrams best represents a strong acid, such as HCl, dissolved in water? Which represents a weak acid? Which represents a very weak acid? (The hydrated proton is shown as a...
-
A gaseous sample of a substance is cooled at constant pressure. Which of the following diagrams best represents the situation if the final temperature is (a) Above the boiling point of the substance...
-
A potential difference of 1.20 V will be applied to a 33.0 m length of 18-gauge copper wire (diameter = 0.0400 in.). Calculate (a) The current, (b) The magnitude of the current density, (c) The...
-
The Davidson Corporation's balance sheet and income statement are provided here. Davidson Corporation: Income Statement for Year Ending December 31, 2012 (Millions of Dollars)...
-
In a sample of 1000 randomly selected consumers who had opportunities to send in a rebate claim form after purchasing a product, 250 of these people said they never did so ("Rebates: Get What You...
-
Describe the trade-off between model complexity and prediction error.
-
Panoramic Inc. had a beginning balance of $2,000 in its Accounts Receivable account. The ending balance of Accounts Receivable was $2,400. During the period, Panoramic recognized $40,000 of revenue...
-
A company has two products: At and B2. It uses active based costing and has prepared the following anys showing budgeted cost and activity for each of the activities Bodited Activity Activity...
-
Madrid FC own land in the metropolitan area of Madrid. They would like to build a sports complex which would include state-of the art training facilities for elite athletes. Your consultancy office...
-
As a tree respires, it takes in carbon dioxide, CO 2 , and water vapor, H 2 O, from the air, while also releasing oxygen, O 2 . Does the tree lose or gain weight as it respires? Explain.
-
A beam of protons and a beam of neutrons of the same energy are both harmful to living tissue. The beam of neutrons, however, is less harmful. Suggest why.
-
If the bond of the previous problem paid 10 percent interest per year ($100), at what price would it sell? Data from previous problem What is the present value of $1,000 due in ten years, discounted...
-
Nelsie Corporation has an outstanding 60-day 6% note receivable amounting to P 15,000 dated December of the ne year. The company is using the calendar year in preparing its financial statements. What...
-
Which resource is the bottleneck? What is the overall capacity of the orthopedist's office in patients/hour?
-
What are the comprehensive strategic implentation issues of Kmart with reference
-
1. Create both the written plan and the educational material to help African American women age 65+ control high blood pressure, take the special circumstances into consideration for the plan. 2. For...
-
Write down D & S equations for wireless phones; include two exogenous variables in each equation.
-
What requirements must be met to record an asset?
-
A consumer magazine is evaluating five brands of trash compactors for their effectiveness in reducing the volume of typical household products that are discarded. In the experiment, each block...
-
In cylindrical coordinates, let = 0 for < 1 mm, = 2 sin(2000 ) nC/m 3 for 1 mm < < 1.5 mm, and = 0 for > 1.5 mm. Find D everywhere.
-
The line segment x = 0,1 y 1, z = 1, carries a linear charge density L = |y| C/m. Let z = 0 be a conducting plane and determine the surface charge density at: (a) (0, 0, 0); (b) (0, 1, 0).
-
Atomic hydrogen contains 5.5 10 25 atoms/m 3 at a certain temperature and pressure. When an electric field of 4 kV/m is applied, each dipole formed by the electron and positive nucleus has an...
-
Question 24 Not yet answered Marked out of 1.00 P Flag question Muscat LLC's current assets and current liabilities are OMR 258,000 and OMR 192,000, respectively. In the year 2020, the company earned...
-
Question 24 Miami Company sold merchandise for which it received $710,400, including sales and excise taxes. All of the firms sales are subject to a 6% sales tax but only 50% of sales are subject to...
-
f the IRS intends to close a Taxpayer Assistance Center, they must notify the public at least _____ days in advance of the closure date. 14 30 60 90

Study smarter with the SolutionInn App


