Speculate on why chlorine is stored as a liquid rather than being purchased and stored as a
Question:
Speculate on why chlorine is stored as a liquid rather than being purchased and stored as a gas at the absorber temperature (25 °C), when storing it as a gas would eliminate the need for the chlorine vaporizer and possibly for the heat exchanger in the chlorine feed line.
This problem deal with the chlorine absorber, depicted in the following flowchart.
![Pc, (atm) - PH2olatm) 25°C, Platm) mo(kg/h) 0.10 kg PVC(s)/kg 0.90 kg H,0(1)/kg m,(kg polymer/h) x,(kg Cl,/kg) (1-x,(kg PVC/kg) Vapor Slurry m,(kg liquid/h) x,(kg Cl,/kg) (1 - x,Ikg H20(1)/kg] 25°C, P(atm) ma,Ikg Cl,(g)/h]](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1594/2/8/1/1045f06cc90e61421594281103315.jpg)
The chlorine fed to the tank dissolves in both the liquid phase and the polymer phase. The slurry inside the tank is well mixed, so that its composition is the same as the composition of the exiting stream. The head space above the slurry contains chlorine vapor in equilibrium with the dissolved chlorine in both condensed phases and water vapor in equilibrium with the liquid water in the aqueous phase. The condition inside the absorber is shown schematically in the following diagram:
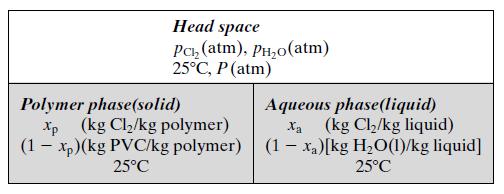
The following physical property data pertain to this system:
• The solubilities of Cl in PVC and in water are correlated by a form of Henry’s law:
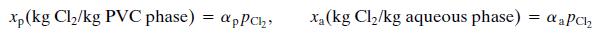
where pCl2 is the partial pressure of chlorine in ht head space. The coefficients αp and αa depend on the system temperature.
• The solubility data shown below have been obtained for chlorine in PVC at 25°C:
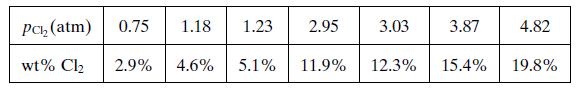
• At 25°C, chlorine gas is 2.68 times more soluble in PVC than in water for any chlorine partial pressure.
• Raoult’s law provides a good correlation of the equilibrium between liquid water and water vapor in the system.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





