Use a commercial flowchart simulation program such as HYSYS or ASPEN to simulate the ammonium nitrate manufacturing
Question:
Use a commercial flowchart simulation program such as HYSYS or ASPEN to simulate the ammonium nitrate manufacturing process described in Example 10.3-3.
Example 10.3-3
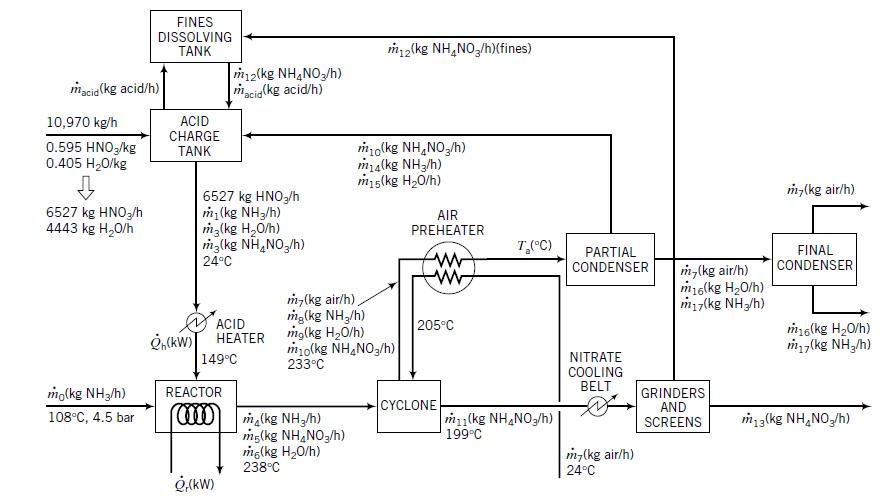
Transcribed Image Text:
FINES DISSOLVING TANK m2(kg NH,NO,/h)(fines) m12(kg NH,NO,/h) macig(kg acid/h) macia (kg acid/h) 10,970 kg/h ACID CHARGE 0.595 HNO,/kg 0.405 H20/kg mo(kg NH,NO,/h) ma(kg NH3/h) m15(kg H20/h) TANK m,(kg air/h) 6527 kg HNO3/h 4443 kg H20/h 6527 kg HNO,/h m (kg NH3/h) m3(kg H,0/h) m3(kg NH,NO,/h) AIR PREHEATER T,(C) PARTIAL CONDENSER FINAL CONDENSER 24°C m,(kg air/h) m16(kg H20/h) miy(kg NH/h) m,(kg air/h), mg(kg NH,/h) mg(kg H20/h) m10(kg NH,NO3/h) 233°C ACID НЕАTER 205°C m6(kg H20/h) m (kg NH,/h) NITRATE COOLING BELT 149°C mo(kg NH3/h) REACTOR GRINDERS AND SCREENS CYCLONE m1(kg NH,NO3/h)| 199°C 108°C, 4.5 bar ma(kg NH,/h) mg(kg NH,NO,/h) mg(kg H20/h) 238°C m3(kg NH,NO,/h) m-(kg air/h) 24°C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau
Question Posted:
Students also viewed these Business questions
-
Repeat Exercise 5 assuming that the size of each batch is 100 instead of 10,000. Compare the control chart to the one found for Exercise 5. Comment on the general quality of the manufacturing process...
-
Ammonium nitrate, NH4NO3, is used as a nitrogen fertilizer and in explosives. What is the molar mass of NH4NO3?
-
The throttle process described in Example 6.5 is an irreversible process. Find the entropy generation per kg ammonia in the throttling process.
-
What do you think people would say about Corrie from the few quotes we have from her book? What was her personality like? Do you think she handled her incarceration differently than Elie Wiesel?...
-
(a) At 800 K the equilibrium constant for I2(g) 2 I(g) is Kc = 3.1 Ã 10-5. If an equilibrium mixture in a 10.0-L vessel contains 2.67 Ã 10-2 g of I(g), how many grams of I2 are in the...
-
Overhead analysis. Armstrong Corporation uses standard costing. The following information is for 2007: Static-budget machine-hours 33,000 Fixed overhead budget costs $ 5,940,000 Fixed overhead actual...
-
8. How does a proprietary fund statement of cash flows differ from a commercial enterprises statement of cash flows?
-
The widths (in meters) of a kidney-shaped swimming pool were measured at 2-meter intervals as indicated in the figure. Use the Midpoint Rule to estimate the area of the pool. 5.6 5.0 6.8 4.8 4.8 7.2...
-
2. Matthew (48 at year-end) develops cutting-edge technology for SV Incorporated, located in Silicon Valley. In 2023, Matthew participates in SV's money purchase pension plan (a defined contribution...
-
The accounts of Pyle Company and its subsidiary, Stern Company, are summarized below as of December 31, 2011: Pyle Company made the following open-market purchase and sale of Stern Company common...
-
You are to write the code for a convergence module that can deal with one to three tear stream variables using theWegstein algorithm, as outlined in Appendix A.2. The object is to determine the...
-
On an oversized page, draw a flowchart for this process. Label each stream with an identifying symbol (e.g., S l , S 2 , S 3 , ...) and known information about what the stream is and/or what it...
-
In the pyrimidine degradative pathway, all pyrimidines undergo conversion to uracil, which undergoes an NADPH-dependent reduction. Show plausible reactions leading from cytidine, cytosine, and...
-
Your introduction needs to include the following. o Include a clear definition of unemployment and inflation and how and why they occur and rise in the economy. o Briefly provide your understanding...
-
Questions: 1. What strategies can be employed to foster a sense of inclusion and belonging within teams, and what are the potential benefits of doing so? 2. How can a team be successful? 3. What is...
-
Critical reflection involves closely examining events and experiences from different perspectives to inform future practice. In a few paragraphs, explain - Why educators should regularly reflect on...
-
What resources does the school or school district provide to teachers to promote diversity, equity, and inclusion? What are some of the strengths and shortcomings of the school's policies on...
-
Select FOUR companies listed on the UK Stock Exchange. Chose two companies from one industry sector and two other companies from another industry sector. By using the most recent three years'...
-
A promising new technology for generating electricity uses microbial fuel cells (MFCs)a product of natural human wastewaters. Over the past several years, research in employing MFCs for this purpose...
-
Juanita owns a home in Richardson, TX. She purchases a Homeowners Policy (HO-3) from Farm State Ins. Co. The policy provides $100,000 in liability coverage (coverage E) and $5,000 in Med Pay coverage...
-
If the current in an electric conductor is 2.4 A, how many coulombs of charge pass any point in a 30 second interval?
-
Determine the time interval required for a 12-A battery charger to deliver 4800 C.
-
A lightning bolt carrying 30,000 A lasts for 50 microseconds. If the lightning strikes an airplane flying at 20,000 feet, what is the charge deposited on the plane?
-
You are the digital marketing director for High West fashions, a regional clothing company that specializes in custom t-shirts. Your company has decided to launch an online advertising campaign that...
-
In-the-money put options will automatically get exercised at the expiration. True OR False
-
Which of the following examples of business-use property is NOT eligible for Section 1231 treatment when sold at a gain? * Sale of land held for three years. Net gain from a casualty gain on a dump...

Study smarter with the SolutionInn App



