The chemistry of most metabolic reactions was deciphered by synthesizing metabolites containing atoms that are different isotopes
Question:
The chemistry of most metabolic reactions was deciphered by synthesizing metabolites containing atoms that are different isotopes from those occurring naturally. The products of reactions starting with isotopically labeled metabolites can be analyzed to determine precisely which atoms in the products are derived from which atoms in the starting material. The methods of detection exploit, for example, the fact that different isotopes have different masses that can be distinguished using biophysical techniques such as mass spectrometry. Moreover, some isotopes are radioactive and can therefore be readily recognized with electronic counters or photographic film that becomes exposed by radiation.
A. Assume that pyruvate containing radioactive 14C in its carboxyl group is added to a cell extract that can support oxidative phosphorylation. Which of the molecules produced should contain the vast majority of the 14C that was added?
B. Assume that oxaloacetate containing radioactive 14C in its keto group (refer to Panel 13–2, pp. 442 443) is added to the extract. Where should the 14C atom be located after precisely one turn of the citric acid cycle.
Panel 13–2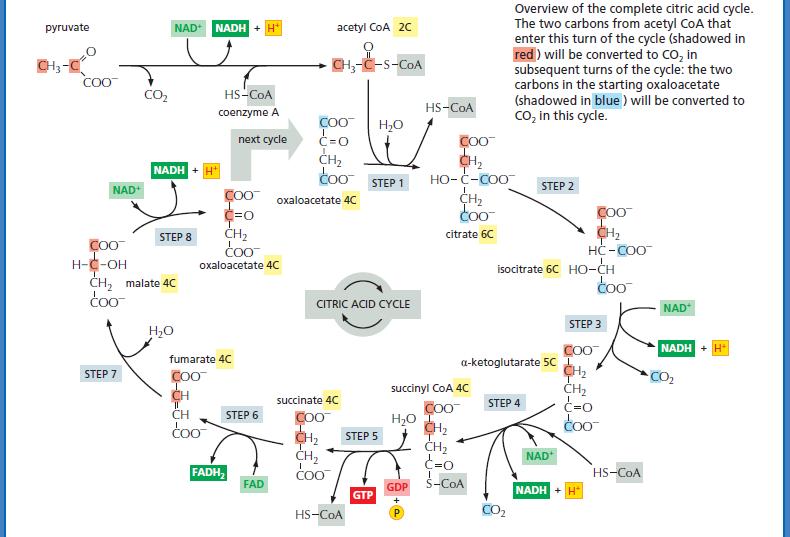
Step by Step Answer:

Essential Cell Biology
ISBN: 9780393680362
5th Edition
Authors: Bruce Alberts, Karen Hopkin, Alexander Johnson, David Morgan, Martin Raff, Keith Roberts, Peter Walter





