Show that the field on the x-axis for the dipole of Example 20.5 is given by Equation
Question:
Show that the field on the x-axis for the dipole of Example 20.5 is given by Equation 20.6b, for |x|>>a.
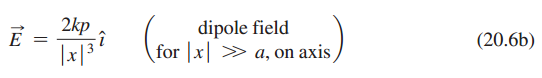
Transcribed Image Text:
2kp E dipole field for x > a, on axis (20.6b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
Kq EA AP EB E axial Kq Kq BP xa i E...View the full answer

Answered By

Rajeev Ranjan
I am in this profession of teaching in a Dayanidhi international school for about 03 years. Every time whenever I am going to teach a class as a part of this noble profession .....I am learning also. I am trying consistently ....how to represent, interpret, summarize and rethink about the whole concept I wish to teach or rather inspire my students in a specific period of time assigned to me.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The Temkin rate equation for NH3 synthesis is based on the 2-step sequence provided in Illustration 8.1 and it is given by equation 2 in that Illustration. With the information given there, i.e., m =...
-
The red wave shown in Fig. E1.5 is given by u = 5cos4px (V). What expression is applicable to (a) the blue wave and (b) the green wave? o (volts) 3.52 V 1.01 V 0.25/ 0.5 10.75/ 1.0 -5 Figure E1.5
-
The location of the sliding bar in Figure 9.5 is given by x = 5t + 2t 3 , and the separation of the two rails is 20 cm. Let B = 0.8x 2 a z T. Find the voltmeter reading at (a) t = 0.4 s; (b) x = 0.6...
-
On December 31, 2019, Metlock Inc. borrowed $3,300,000 at 13% payable annually to finance the construction of a new building. In 2020, the company made the following expenditures related to this...
-
A firefighter, 60.0 m away from a burning building, directs a stream of water from a ground-level fire hose at an angle of 37.0 above the horizontal. If the water leaves the hose at 40.3 m/s, which...
-
What advice would you give Suzanne Howard and her team for improving the employee involvement climate at the Lubbock plant? LO.1
-
Check that the determinant of diagonal and triangular matrices is the product of elements on the diagonal.
-
Both Seco Inc. and Hillsborough Corp. have 100,000 shares of no-par common stock outstanding. World Inc. acquired 10,000 shares of Seco stock for $6 per share and 30,000 shares of Hillsborough stock...
-
What's the present value of $9,500 discounted back 5 years if the appropriate interest rate is 4.5%, compounded semiannually?
-
Frank is looking at a new sausage system with an installed cost of $560,000. This cost will be depreciated straight line to zero over the projects five-year life, at the end of which the sausages...
-
A solid sphere 10 cm in radius carries a 40C charge distributed uniformly throughout its volume. Its surrounded by a concentric shell 20 cm in radius, also uniformly charged with 40 C. Find the...
-
Two identical dipoles, each of charge q and separation a, are a distance x apart, as shown in Fig. 20.31.(a) By considering forces between pairs of charges in the different dipoles, calculate the...
-
Demand for walnut fudge ice cream at the Sweet Cream Dairy can be approximated by a normal distribution with a mean of 21 gallons per week and a standard deviation of 3.5 gallons per week. The new...
-
The government is issuing $100 million in 10 year debt and receives the following bids. $25 million is reserved for non-competitive tenders. At what yield will the non-competitive tenders be issued...
-
Carrie enjoyed observing wildlife in natural habitats. She wanted to be able to hide at a distance but observe wildlife close up in a variety of circumstances. She visited five different shops and...
-
The operating budget for a certain company shows a net income of $350,000. To achieve this, the company is targeting sales of $637,000, variable costs of $280,280, and fixed costs of $6,720. Compute...
-
What is the output? for (i=0; i
-
a) For silicon, if EG decreases by 0.078 eV, by what fraction does n; increase (assume T is constant at 300K)? b) If the temperature rises from 300K to 600K, by what additional fraction does n;...
-
A baryon decays strongly into + and + , but not into 0 0 nor into + + , even if all are energetically possible. (1) What can you tell about its isospin? (2) You should check your conclusion...
-
What steps must a business take to implement a program of social responsibility?
-
21.05 g of steam at 373 K is added to 415 g of H 2 O(l) at 298 K at a constant pressure of 1 bar. Is the final state of the system steam or liquid water? Calculate S for the process.
-
A van der Waals gas has a value of z = 1.00061 at 410. K and 1 bar and the Boyle temperature of the gas is 195 K. Because the density is low, you can calculate V m from the ideal gas law. Use this...
-
A sample of Na2SO4(s) is dissolved in 225 g of water at 298 K such that the solution is 0.325 molar in Na 2 SO 4 . A temperature rise of 0.146C is observed. The calorimeter constant is 330. J K 1 ....
-
Derek plans to retire on his 65th birthday. However, he plans to work part-time until he turns 71.00. During these years of part-time work, he will neither make deposits to nor take withdrawals from...
-
Penske Ltd has a standard deviation of returns of 18% and a correlation with the market portfolio of 0.8. The market portfolios expected return is 14%, its standard deviation of returns is 12%, and...
-
What is the quoted price of a bond maturing in 12 years with a coupon rate of 9 percent, paid semiannually, that has a YTM of 13 percent? (Please round to the nearest hundredth)

Study smarter with the SolutionInn App


