The table below shows the wavelengths of photons emitted when identical molecules drop from the lth rotational
Question:
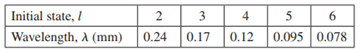
The table below shows the wavelengths of photons emitted when identical molecules drop from the lth rotational level to the (l - 1)th level. Find quantities that, when plotted, should yield a straight line. Make your plot, determine a best-fit line, and use the result to find the rotational inertia of the molecules.
Transcribed Image Text:
Initial state, I 3 4 5 Wavelength, A (mm) 0.24 0.17 0.12 0.095 0.078
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
This table shows the wavelength of photons emitted when identical molecules drop from the lth rotati...View the full answer

Answered By

Akshay Shete
I have extensive experience as a tutor, both online and in-person. I have worked with students of all ages and abilities, and am skilled at adapting my teaching style to meet the needs of each individual student. I have a strong background in a variety of subjects, including math, science, and English, and am able to break down complex concepts in a way that is easy for students to understand. In addition to my subject matter expertise, I am also a patient and supportive teacher, and am committed to helping my students succeed. Whether I am working with a struggling student who needs extra help to catch up, or an advanced student looking to get ahead, I am able to provide the guidance and support they need to reach their goals. Overall, my hands-on experience as a tutor has prepared me to be a confident and effective teacher, and I am excited to use my skills to help students succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Measurements of the potential at points on the axis of a charged disk are given in the two tables below, one for measurements made close to the disk and the other for measurements made far away. In...
-
The table below lists the stopping potential as a function of wavelength in a photoelectric effect experiment. Determine quantities to plot that should yield a straight line. Make your plot,...
-
The table below lists the wavelengths emitted as electrons in identical square-well potentials drop from various states n to the ground state. Determine a quantity that, when you plot l against it,...
-
1. The following are selected accounts taken from the adjusted trial balances of the Purell Merchandise company on December 31, 2021: Inventory, January 1, 2021 Selling expenses Loss on sale of...
-
Give a substitutive name for each of the following compounds. (a) CH3CHT-O-CH'CH'-OH (b)
-
This question is about two transition metals, hafnium (Hf) and zirconium (Zr). a. Hafnium forms a peroxide whose formula can be written as HfO 3 .2H 2 O. Use the A r values below to calculate the...
-
2. Determine the total budgeted cost for the project.
-
Use the Amazon.com financial statements in Appendix A at the end of this book to answer the following questions. Requirements 1. Show how Amazon computed basic earnings per share of $2.08 for 2009....
-
X Co. adopts a plan of complete liquidation and makes the following pro rata distributions to its shareholders (assume all are individuals): A Cash $70,000 B Inventory FMV $20,000 Basis $20,000...
-
ASAP Delivery is a small company that transports business packages between San Francisco and Los Angeles. It operates a fleet of small vans that moves packages to and from a central depot within each...
-
Use Equation 37.5 to calculate the average energy of a conduction electron at T = 0 in terms of the Fermi energy. (27/2Tm 32 VE g(E) = (37.5) h3
-
Photovoltaic (PV) cells convert sunlight energy directly into electricity, with no moving parts (recall Fig. 37.21). In a PV cell, photons incident on a semiconductor PN junction promote electrons to...
-
(a) Why is it illogical to use a thin stationary phase (0.2 m) in a wide-bore (0.53-mm) open tubular column? (b) Consider a narrow-bore (0.25 mm diameter), thin-film (0.10 m) column with 5 000 plates...
-
You have just come into an inheritance of $25,000 from a distant relative, and you want to invest it for the long term. Provide an investment portfolio that includes five different stocks. Report the...
-
As the human resource manager, how would you evaluate the training needs of your staff? How can you ensure that the training you would provide is effective? What data might be used to make your...
-
MARYLAND CORPORATION manufactures three liquid products - Alpha, Beta and Gamma using a joint process with direct materials, direct labor and overhead totaling $560,000 per batch. In addition, the...
-
Three common organizational structures. Mention one organization for each organizational structure which is following a specific organizational structure. Also, provide support to your answer by...
-
You are a retail manager at Kitchen Nightmare, a relatively new store at the mall that sells mostly items for kitchens, like forks, oven mitts, etc.. You have been open since the fall of 2021 and...
-
Solve the equations AX = Y, where: [ 13 -8 -3] 20 X1 3D|-8 10 -1|, %3D| -5|, X-| x, [-3 -1 11] X3
-
What is the difference between direct materials and indirect materials?
-
The triangular plate is fixed at its base, and its apex A is given a horizontal displacement of 5 mm. Determine the average normal strain ε x along the x axis. 45 800 mm 45 A' 5 mm 45%...
-
The stressstrain diagram for polyethylene, which is used to sheath coaxial cables, is determined from testing a specimen that has a gage length of 10 in. If a load P on the specimen develops a strain...
-
Determine the average normal strain that occurs along the diagonals AC and DB. 5 mm, 2 mm 4 mm 2 mm 300 mm T2 mm 400 mm 3 mm
-
Effect of stock split Willeys Grill & Restaurant Corporation wholesales ovens and ranges to restaurants throughout the Southwest. Willeys Grill & Restaurant, which had 33,000 shares of common stock...
-
CAPITAL BUDGETING CRITERIA: MUTUALLY EXCLUSIVE PROJECTS A firm with a WACC of 10% is considering the following mutually exclusive projects: 0 1 2 3 4 5 Project 1 -$450 $55 $55 $55 $190 $190 Project 2...
-
The main purpose of a cash budget is to forecast the company's a. liquidity . b. insolvency. c. profitability. d. net income.

Study smarter with the SolutionInn App


