Today, uranium-235 comprises only 0.72% of natural uranium; essentially all the rest is U-238. Use the half-lives
Question:
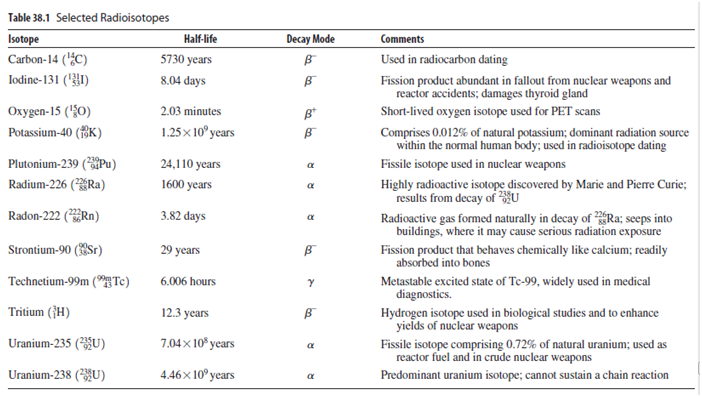
Today, uranium-235 comprises only 0.72% of natural uranium; essentially all the rest is U-238. Use the half-lives in Table 38.1 to determine the percentage of uranium-235 when Earth formed about 4.5 billion years ago.
Transcribed Image Text:
Table 38.1 Selected Radioisotopes Decay Mode Isotope Carbon-14 (¿C) Half-life Comments 5730 years Used in radiocarbon dating Iodine-131 ('1) 8.04 days Fission product abundant in fallout from nuclear weapons and reactor accidents; damages thyroid gland Охудеn-15 ({0) 2.03 minutes B* Short-lived oxygen isotope used for PET scans 1.25x 10° years Comprises 0.012% of natural potassium; dominant radiation source within the normal human body; used in radioisotope dating Potassium-40 (fK) Plutonium-239 (Pu) 24,110 years Fissile isotope used in nuclear weapons a Radium-226 (*ÝRa) 1600 years Highly radioactive isotope discovered by Marie and Pierre Curie; results from decay of HU Radioactive gas formed naturally in decay of Ra; seeps into buildings, where it may cause serious radiation exposure Fission product that behaves chemically like calcium; readily Radon-222 (Rn) 3.82 days Strontium-90 (Sr) 29 years absorbed into bones Technetium-99m ("HTc) 6.006 hours Metastable excited state of Te-99, widely used in medical diagnostics. Hydrogen isotope used in biological studies and to enhance yields of nuclear weapons Fissile isotope comprising 0.72% of natural uranium; used as reactor fuel and in crude nuclear weapons Tritium (}H) 12.3 years Uranium-235 (U) 7.04× 10* years Uranium-238 (U) 4.46× 10º years Predominant uranium isotope; cannot sustain a chain reaction
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
According to the NEA identified uranium resources total 55 ...View the full answer

Answered By

Dudhat Vaidehi
I tutored mostly elementary school students privately after school and during the summer. We met in their homes or at the public library. I charged an hourly fee, and I provided any necessary materials.
Having taught in special education in two local schools for many years meant that I had contact with a lot of parents of special needs students. I never had to advertise — word of mouth was how most folks knew of me. At one point I did have a website, but didn't utilize it much. I stayed very busy, especially in the summers, and always had a full schedule. I typically met with each student's teacher in order to get an idea of what the focus of my instruction/remediation should be. Becoming familiar with the student's learning style(s) was also very helpful. Often parents would share records and test results with me. After each tutoring session, I documented the student’s progress and gave parents written updates, as well as phone calls or emails as needed.
While my students and I certainly utilized technology and the internet often during our sessions, I never tutored online or for any tutoring company, so am not familiar with the curriculums or methods used in those settings.
Tutoring one on one was very enjoyable and rewarding. My students and I had fun, and grew quite fond of one another. The extra income was a bonus. I had to retire from tutoring due to a physically handicapping disease, and miss my students very much.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Naturally occurring uranium is composed mostly of 238U and 235U, with relative abundances of 99.28% and 0.72%, respectively. The half-life for 238U is 4.5 X 109 years, and the half-life for is 7.1 X...
-
In 1972, a worker at a nuclear fuel plant in France found that uranium from a mine in Oklo, in the African Republic of Gabon, had less U-235 than the normal 0.7% a quantity known from meteorites and...
-
Almost all of naturally occurring uranium is 238/92U with a half-life of 4.468 x 109 yr. Most of the rest of natural uranium is 235/92U with a half-life of 7.038 x 108yr. Today a sample contains...
-
4. Papo and Pepe are two barbers from a small barbershop. Theyhave their two court chairs plus two waiting chairs. The followingresults were found: P0 = 1/16 P1 = 4/16 P2 = 6/16 P3 = 4/16 a. What is...
-
Draw the structures of the following compounds. (Some parts may have more than one correct answer.) (a) An achiral trimethylcyclohexane with two chair forms that are conformational diastereomers. (b)...
-
A manufacturer provided the following information: $ Prime cost .............................................. 132,000 Factory overheads .................................. 17,000 Opening work in...
-
What has this case description got to do with data mining? LO.1
-
A firm uses a single plant with costs C = 160 + 16Q + .1Q2 and faces the price equation P = 96 - .4Q. a. Find the firms profit-maximizing price and quantity. What is its profit? b. The firms...
-
Following a strategy of product differentiation, R2D2 Corporation makes a high-end computer monitor, BB8. R2D2 Corporation presents the following data for the years 1 and 2: Units of BB8 produced and...
-
George Pharmacy is a pharmaceutical salesman who has been very successful at his job in the last few years. Unfortunately, his family life has not been very happy. Three years ago, his only child,...
-
Brachytherapy is a cancer treatment involving implantation of radioactive seeds at the tumor site. Iridium-192, often used for cancers of the head and neck, undergoes beta decay by electron capture...
-
Youre a geologist assessing underground sites for nuclear waste storage. A ruling by the U.S. Environmental Protection Agency suggests that waste-storage facilities should be designed for a million...
-
1 Think of products and services that are currently successful, determine the innovations that created that success and categorise them using the 4Ps model.
-
What amount of cash payments to suppliers will be reported by Indigo Company for the year ended December 31, 2024?
-
Moving Inc. wants to develop an activity flexible budget for the activity of moving materials. Moving Inc. uses forklifts to move materials from receiving to storeroom and then to production. The...
-
We are in the tail end of Quarter 3 earnings reporting season in the U.S. markets. Roughly 60 percent of companies that have reported their Q3 earnings so far have reported negative earnings relative...
-
Below is a running shock tube illustration. 0.1 0.0 | 0.0 4 4 Diaphragm 1 0.5 Image: Shock tube Initial setup 1 3 2 1 Expansion Head Expansion Tail Slip Shock Surface Image: Running Shock Tube...
-
As you may remember, Holiday Tree Services, Inc. (HTS) has recently entered into a contract with Delish Burger (Delish), whereby HTS is to supply and decorate a Christmas tree in each of Delish...
-
Fill in the template file below. A B F G K RETURN DATA: 10 STOCKS AND SP500 1 1 2 3 4 6 7 8. 10 11 Whole Hewlett Goldman Johnson- General Johnson Electric 3 Apple Google Foods Seagate Comcast Merck...
-
9.Consider the reaction 3NO2(g)+H2O=2HNO3(aq)+NO(g) where Delta H=-137 kJ.How many kilojoules are released when 92.3g of NO2 reacts?
-
The post is made from 606l-T6 aluminum and has a diameter of 50 mm. It is fixed supported at A and B, and at its center C there is a coiled spring attached to the rigid collar. If the spring is...
-
The post is made from 606l-T6 aluminum and has a diameter of 50 mm. It is fixed supported at A and B, and at its center C there is a coiled spring attached to the rigid collar. If the spring is...
-
The A-36 steel wires AB and AD each have a diameter of 2 mm and the unloaded lengths of each wire are LAC = 1.60 m and LAB = LAD = 2.00 m. Determine the required diameter of wire AC so that each wire...
-
On February 1, 2021, Arrow Construction Company entered into a three-year construction contract to build a bridge for a price of $8,600,000. During 2021, costs of $2,200,000 were incurred with...
-
Salespersons' Report and Analysis Walthman Industries Inc. employs seven salespersons to sell and distribute its product throughout the state. Data taken from reports received from the salespersons...
-
Stockholders do not have the power to bind the corporation to contracts. This is referred to as lack of mutual agency. True false question. True False

Study smarter with the SolutionInn App


