With sufficient energy, it?s possible to eject an electron from an inner atomic orbital. A higher-energy electron
Question:
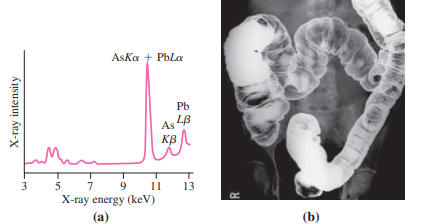
With sufficient energy, it?s possible to eject an electron from an inner atomic orbital. A higher-energy electron will then drop into the unoccupied state, emitting a photon with energy equal to the difference between the two levels. For inner-shell electrons, photon energies are in the keV range, putting them in the X-ray region of the spectrum. These characteristic X rays are labeled with the letter indicating the shell to which the electron drops, followed by a Greek letter indicating the higher level from which it drops; thus Ka designates a transition from the L shell to the K shell. Characteristic X rays provide scientists and physicians with an important diagnostic tool. Environmental scientists bombard pollution samples with high-energy electrons, knocking out inner-shell electrons and thus producing X-ray spectra that help identify contaminants (Fig. 36.20a). Geologists do the same with rocks. Medical radiologists reverse the process, exploiting the fact that X rays cause inner-shell transitions as well as complete ejection of inner-shell electrons. In particular, radiologists use the element barium in this way to produce high-contrast X-ray images of the intestinal tract (Fig. 36.20b).
?
Elements A and B have atomic numbers ZA and ZB = 2ZA. How do you expect element B?s K? X-ray energy to compare with that of element A?a. B?s K? energy should be about one-fourth that of A.b. B?s K? energy should be about half that of A.c. B?s K? energy should be about twice that of A.d. B?s K? energy should be about four times that of A.
Step by Step Answer:






