The ideal gas equation of state is very simple, but its range of applicability is limited. A
Question:
The ideal gas equation of state is very simple, but its range of applicability is limited. A more accurate but complicated equation is the Van der Waals equation of state given by
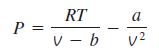
where a and b are constants depending on critical pressure and temperatures of the gas. Predict the coefficient of compressibility of nitrogen gas at T = 175 K and ν = 0.00375 m3/kg, assuming the nitrogen to obey the Van der Waals equation of state. Compare your result with the ideal gas value. Take a = 0.175 m6·kPa/kg2 and b = 0.00138 m3/kg for the given conditions. The experimentally measured pressure of nitrogen is 10,000 kPa.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fluid Mechanics Fundamentals And Applications
ISBN: 9780073380322
3rd Edition
Authors: Yunus Cengel, John Cimbala
Question Posted:




