Ammonia (NH 3 ) in air is being absorbed into water within the enclosed tank shown in
Question:
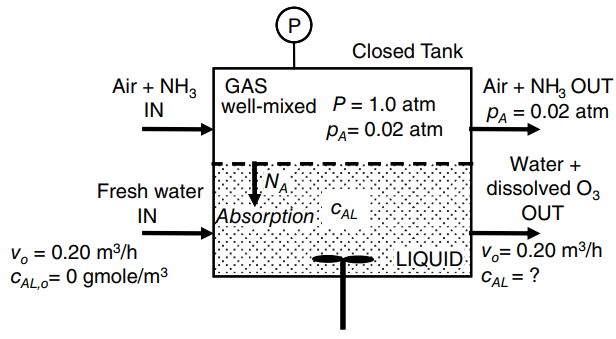
a. Develop a material balance equation for NH3 (solute A). Then determine cAL, the concentration of dissolved NH3 in the outlet liquid stream. In material balances involving inter phase mass-transfer, base the material balance on one phase. For this process, consider a material balance on NH3 based on the liquid phase.
b. Determine pA,i the partial pressure of NH3 at the gas€“liquid interface, and cAL,i, the dissolved NH3 concentration at the liquid side of the gas€“liquid interface.
c. Determine WA, the total rate of ammonia transfer.
d. In the above system, the flux NA would increase by increasing which of the following: the liquid volume level in the tank at fixed surface area; the agitation intensity of the bulk liquid; the agitation intensity of the bulk gas; the inlet liquid volumetric flow rate; the system temperature.
The word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





