A compound has a molecular mass of 120 g/mol, and the information in the table below is
Question:
A compound has a molecular mass of 120 g/mol, and the information in the table below is the only other data available for a compound. Fill in all of the empty cells with your best estimate of the value. Explain any assumptions or approximations you make.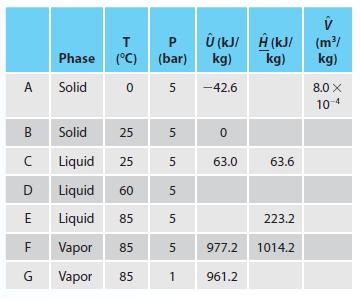
Transcribed Image Text:
A B с D E F G T Phase (°C) (°C) Solid 0 Solid 25 Liquid 25 Liquid 60 Liquid 85 Vapor 85 Vapor 85 P (bar) 5 5 сл 5 5 5 5 1 сл Û (kJ/ kg) -42.6 0 63.0 977.2 961.2 V Ĥ (kJ/(m²/ kg) kg) 63.6 223.2 1014.2 8.0 X 10-4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Here are my best estimates for the empty cells in the tablebased on the information provided Phase T ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Given below are the zeros and its multiplicities, write the function in factored form Zeros: 4 mult 1 -1 mult 2 -5 mult 3
-
THIRD AVENUE SOFTWARE HEALTH-CARE APP PROJECT This case is new for the ninth edition of Information Technology Project Management . The case provides an opportunity to apply agile and Scrum...
-
State whether or not each of the following events would result in a liability being recognised in the accounts at 30 June. 1. Taxes for the year ended 30 June, which are not payable until October. 2....
-
On January 1, 2010, Turner Construction Company agreed to construct an observatory for Dartmouth College for $120 million. Dartmouth College must pay $30 million upon signing and $30 million at the...
-
It seems logical that restaurant chains with more units (restaurants) would have greater sales. This assumption is mitigated, however, by several possibilities: some units may be more profitable than...
-
Discuss the key issues in international industrial relations and the policies and practices of multinationals. LO9
-
Explain the theory behind the free cash flows valuation approach. Why are free cash flows value-relevant to common equity shareholders when they are not cash flows to those shareholders, but rather...
-
Drongo Corporation's 3-year bonds currently yleld 4.7 percent and have an inflation premium of 2%. The real risk-free rate of Interest, ris 1.3 percent and is assumed to be constant. The maturity...
-
10 m 3 of saturated steam at T = 150C is mixed with 0.1 m 3 of saturated liquid water at T = 150C. How many total kilograms of H 2 O does the mixture contain?
-
Model water using the van der Waals equation of state with a = 5.53 10 6 bar cm 6 /mol 2 and b = 30.48 cm 3 /mol (The values of the van der Waals a and b were determined using the method...
-
Which of the following statements regarding insurance bonding is FALSE? a) Insurance bonding doesn't impact bank credit facilities. b) Insurance doesn't adversely affect the borrower's level of...
-
Describe A demographic profile of the population and community that will be served through the reinvented Human Service program. The description must include all eligibility requirements (i.e.,...
-
You work for a major financial institution. Your branch handles customer calls from a wide variety of individuals. Recently, you've noticed an increase in calls from individuals from African...
-
Pop Company holds 70% of Son Company stock. Pop has sold inventory to Son Company as follows: Percent of Sold Sales Inventory Cost to Price to Held at Year Pop Son Year end 2018 $203,000 $355,000 30%...
-
A B C D E F G H J K L 1 Cost Mortgage Payments 2 Cost Description The upscale hotel's building was acquired for $10 million, leading to monthly mortgage payments of $60,000. Behavior Dollar Amount...
-
What celebrity attributes make for effective celebrity product endorsements? Celebrity testimonials are advertising messages delivered by famous people who say or imply that they use the...
-
a. Propose a mechanism for the following reaction: b. Given that the Ho value for the reaction is -42 kcal/mol and the bond dissociation energies for the C--H, C--Cl and O--H bonds are 101, 82, and...
-
A spacecraft has left the earth and is moving toward Mars. An observer on the earth finds that, relative to measurements made when the spacecraft was at rest, its a. length is shorter b. KE is less...
-
The radius of an input pinion is 3.8 cm, and the radius of an output gear is 11.4 cm. Calculate the velocity and torque ratios of the gearset.
-
The torque ratio of a gearset is 0.75. The pinion gear has 36 teeth and a diametral pitch of 8. Determine the number of teeth on the output gear and the radii of both gears.
-
You are designing a geartrain with three spur gears: one input gear, one idler gear, and one output gear. The diametral pitch for the geartrain is 16. The diameter of the input gear needs to be twice...
-
PLEASE HELP WITH SCHEDULE D, 1125A AND 1120 AND ARE MY CALCULATION RIGHT. Page 2 Form 8949 (2019) Attachment Sequence No. 12A Name(s) shown on return. Name and SSN or taxpayer identification no. not...
-
Using the appropriate interest table, provide the solution to each of the following four questions by computing the unknowns. Click here to view factor tables What is the amount of the payments that...
-
View Policies Current Attempt in Progress Here are comparative statement data for Duke Company and Lord Company, two competitors. All balance sheet data are as of December 31, 2020, and December 31,...

Study smarter with the SolutionInn App


