A mole of gas in a closed system undergoes a fourstep, cyclic process that returns it to
Question:
A mole of gas in a closed system undergoes a fourstep, cyclic process that returns it to the original state 1. The following table gives some data on the steps of the process. Fill in the blanks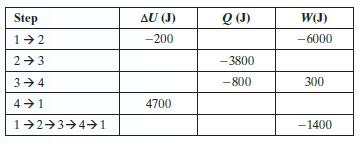
Transcribed Image Text:
Step 1-2 2+3 3-4 4 1 1 2 3 4 1 AU (J) -200 4700 Q (J) -3800 -800 W(J) -6000 300 -1400
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
for step 12 delta Uqw 200Q6000 Q5800 J for step 34 delta Uqw 800300 500 N...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
What are the 4 stages of the Carnot Cycle? Select four answers. Partial credit will be given for correct marks, and negative marks applied for incorrect answers. An overall score lower than zero...
-
One mole of gas in a closed system undergoes a four-step thermodynamic cycle. Use the data given in the following table to determine numerical values fur the missing quantities, i.e., "fill in the...
-
One mole of an ideal gas undergoes an isothermal reversible expansion at 25oC. During this process, the system absorbs 855 J of heat from the surroundings. When this gas is compressed to the original...
-
Calculate the managerial remuneration from the following particulars of Zen Ltd. the company has only one Managing Director. Net Profit Net Profit is calculated after considering the following:...
-
A large manufacturer of truck and car tires recently changed its cost-flow assumption method for inventories at the beginning of 2010. The manufacturer has been in operation for almost 40 years, and...
-
Is it possible to predict the annual number of business bankruptcies by the number of firm births (business starts) in the United States? The following data, published by the U.S. Small Business...
-
Outline key concerns for trade unions. LO9
-
The reaction between ethylene and hydrogen bromide to form ethyl bromide is carried out in a continuous reactor. The product stream is analyzed and found to contain 51.7 mole% C2H5Br and 17.3%HBr....
-
In the variable labor budget, why is it necessary to determine how many RVUs can be handled by full-time employees?
-
A 1 m 3 vessel contains a mixture of saturated liquid water and saturated steam at T = 200C. If the vessel contains 15 kg in total, find the mass of liquid water and the mass of steam.
-
10 m 3 of saturated steam at T = 150C is mixed with 0.1 m 3 of saturated liquid water at T = 150C. How many total kilograms of H 2 O does the mixture contain?
-
There is evidence (Black, Christensen, Joo and Schmardebeck ) suggesting that managers prefer to meet expectations based on neutral reporting of solid operational performance. They also found that,...
-
based on the article How Chili's Is Prepping for Tough Times, Starting With the Fries by Heather Haddon. What is corporate social responsibility, and what is one way that Chili's can better pursue...
-
QUESTION 2 (20 marks) CLO 5 a. Explain what the following ratios indicate to a firm: (i) Acid Test Ratio (ii) Return on Capital Employed (ROCE) (iii) Debtors Collection Period (iv) Working Capital (2...
-
Lazlo s estimates uncollectible accounts to be 0 . 9 % of sales. Its year - end unadjusted trial balance shows Accounts Receivable of $ 1 1 2 , 5 0 0 and sales of $ 9 6 5 , 0 0 0 . If Lazlo s uses...
-
Identify one or two of the best and one or two of the worst work teams on which you served as a member. 1. Identify the top three to five factors that made the team the best or the worst in terms of...
-
ColorCoder is a HousePaint Shop which supplies currently two types of house paints, namely, alpha and beta house paints. The shop is planning to sell a primer (paint base) and the needed paint...
-
A possible alternative mechanism to that shown in Problem 24 for the monochlorination of ethane would involve the following propagation steps: CH3--H + Cl - CH3--Cl + H H + Cl--Cl - H--Cl + Cl How do...
-
Without solving, determine the character of the solutions of each equation in the complex number system. 3x 2 3x + 4 = 0
-
A gasoline-powered engine produces 15 hp as it drives a water pump at a construction site. If the engines speed is 450 rpm, determine the torque T that is transmitted from the output shaft of the...
-
A small automobile engine produces 260 N m of torque at 2100 rpm. Determine the engines power output in the units of kW and hp.
-
A diesel engine for marine propulsion applications produces a maximum power of 900 hp and torque of 5300 N m. Determine the engine speed necessary for this production in rpm.
-
YOUR DECISION: My primary focus is to offer assistance to the crisis team. I will interview all maintenance personnel and document their statements regarding procedures. I will then generate a...
-
What would be some pros and cons of using acutal versus capacity cost driver rates and vice versus. this is the question i am ultimately answering, "Which cost driver rates should be used: actual or...
-
The Auditors Responsibility with Going Concerns Read The auditors consideration of an entitys ability to continue as a going concern. Based on the reading, analyze the auditors responsibility to...

Study smarter with the SolutionInn App


