A quantity of carbon dioxide is confined in a sealed container. For each of the following cases,
Question:
A quantity of carbon dioxide is confined in a sealed container. For each of the following cases, estimate the pressure of the carbon dioxide, using both the ideal gas law and the van der Waals equation of state. The parameters used to model carbon dioxide using the van der Waals equation of state are a = 3.658 × 106 bar · cm6/mol and b = 42.86 cm3/mol.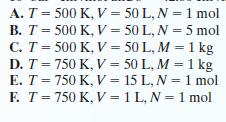
Transcribed Image Text:
A. T = 500 K, V = 50 L, N = 1 mol B. T = 500 K, V = C. T = 500 K, V = D. T = 750 K, V = E. T = 750 K, V = F. T = 750 K, V = 50 L, N = 5 mol 50 L, M = 1 kg 50 L, M = 1 kg 15 L, N = 1 mol 1 L, N = 1 mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To estimate the pressure of carbon dioxide in a sealed container for the given cases using both the ideal gas law and the van der Waals equation of st...View the full answer

Answered By

Mahesh G
I have more than 7 years of experience in teaching physics, mathematics and python programming to more than 600 students including both online and offline tutoring.
I follow the following 7 step fundamental approach towards tutoring.
1. Curiosity, scope, enlightenment of the topic in hand.
2. Problem Definitions and elaboration.
3. Requisite mathematics, analytical abilities and quantitative
aptitude.
4. Preparing Algorithms for problem statement.
5. Concepts with analogies and building algorithm.
6. Introspection and improvising.
7. Daily class wise Cheat sheets(its not cheating) for consolidation.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
The van der Waals equation of state is where a and b are temperature-independent parameters that have different values for each gas. For carbon dioxide, a = 0.3640 Pa m 6 mol 2 and b = 4.267 Ã...
-
The van der Waals equation of state is For carbon dioxide, a = 0.3640 Pa m 6 mol 1 and b = 4.267 Ã 10 5 m 3 mol 1 . Find the pressure of 0.7500 mol of carbon dioxide if V = 0.0242 m 3 and T =...
-
In Sample Exercise 10.16, we found that one mole of Cl2 confined to 22.41 L at 0oC deviated slightly from ideal behavior. Calculate the pressure exerted by 1.00 mol Cl2 confined to a smaller volume,...
-
M1 is a way to measure... a) the level of bank reserves b) a country's money supply c) the level of savings in a country d) a country's economic potential
-
What criteria do you think should be used to measure team performance? What sources should be used for the appraisal? Should individual performance still be measured? Why or why not?
-
Now draw the indifference curve corresponding to a utility level of .05 for an investor with risk aversion coefficient A = 4. Comparing your answer to Problem 6, what do you conclude?
-
What percentage of the CEOs in the survey were married with a stable home life? a. 40 percent c. 80 percent b. 60 percent d. 100 percent
-
Exhaust gases from a wire processing oven are discharged into a tall stack, and the gas and stack surface temperatures at the outlet of the stack must be estimated. Knowledge of the outlet gas...
-
One of X Company's production machines was badly damaged recently. Unfortunately, the machine was purchased just three years earlier for $50,000. The company must either repair the machine or...
-
Element 109, now named meitnerium (in honor of the AustrianSwedish physicist, Lise Meitner [18781968]), was produced in August 1982 by a team at Germanys Institute for Heavy Ion Research. Depict its...
-
The boiler is an important unit operation in the Rankine cycle. This problem further explores the phenomenon of boiling. A. When you are heating water on your stove, before the water reaches 100C,...
-
Determine the damping ratio associated with a second-order system in the standard form of Equation 8.32 that corresponds to a maximum (peak) logarithmic magnitude of \(15.22 \mathrm{~dB}\)....
-
Jimmy Kolop is a manager of a physical therapy department at Bentley Rehab Center. As a unit manager, Jimmy has limitations on exceeding a budget based on a particular item. Bentley Rehab center...
-
Use z scores to compare the given values. Based on sample data, newborn males have weights with a mean of 3233.5 g and a standard deviation of 933.5 g. Newborn females have weights with a mean of...
-
Glycolic acid is produced electrochemically from ethylene glycol under alkaline conditions(naoh). Hydrogen is produced at the cathode, formic acid and oxalic acid are side products Mass balance to...
-
Alves Berhad beroperasi dalam industri elektronik dan komputer. Pengurus Kewangan sedang dalam proses menyediakan penyata kewangan untuk tahun kewangan apabila dia tiba-tiba jatuh sakit. Anda telah...
-
1. First, calculate p, the probability of CHD-1 in each level of BP Status. Take advantage of the fact that the mean of a 0/1 variable is the probability or percentage of observations equaling 1. a....
-
Write the converse and the contrapositive to the following statements. (a) If the measure of angle ABC is 45, then angle ABC is an acute angle. (b) If a < b then a2 < b2.
-
Starr Co. had sales revenue of $540,000 in 2014. Other items recorded during the year were: Cost of goods sold ..................................................... $330,000 Salaries and wages...
-
The parameters and of a substance are reported to be functions of pressure and temperature and are given below: B=1/T, K=1/P. a) Determine the equation of state. Assume that at pressure P. and...
-
a) A tank contains 10,000 kg of xenon at 132 C, 82 bar. The plant supervisor asks you to remove xenon and fill the tank with 10,000 kg of steam at 200 C. What is the pressure in the tank when it is...
-
The boiling point of o-xylene at 1 bar is 139 C. a) What is the state of o-xylene at 0.1 bar, 200 C? b) 100 moles of o-xylene are to be loaded in a tank at 0.1 bar, 200 C. What is the required volume...
-
Aliara Corporation is considering purchasing one of two new machines. Estimates for each machine are as follows: Machine A Machine B Investment $107,800 $156,000 Estimated life 8 years 8 years...
-
XYZ Inc. is a manufacturer of specialized equipment which offers a leasing alternative. Provide journal entries in the books of lessor. The data relative to a typical lease are as follows: 1. The...
-
Inventory information for Part 311 of Sunland Corp. discloses the following information for the month of June. June 1 Balance 303 units @ $14 June 10 Sold 203 units @ $33 11 Purchased 796 units @ $17...

Study smarter with the SolutionInn App


