Example 14-8 illustrated the chlorination of methane to form chloromethane: Example 14-8 also revealed that at equilibrium,
Question:
Example 14-8 illustrated the chlorination of methane to form chloromethane:
![]() Example 14-8 also revealed that at equilibrium, the products were strongly favored. However, the substitution of chlorine for hydrogen can continue in subsequent reactions, forming dichloromethane, chloroform, and carbon tetrachloride:
Example 14-8 also revealed that at equilibrium, the products were strongly favored. However, the substitution of chlorine for hydrogen can continue in subsequent reactions, forming dichloromethane, chloroform, and carbon tetrachloride:
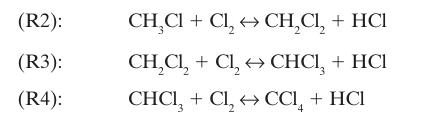
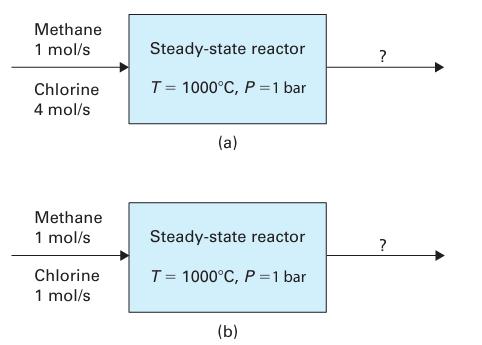
A steady-state reactor is maintained at P = 1 bar and T = 1000°C. Assuming the product stream leaving the reactor is at equilibrium, find its composition if the reactor feed consists of the following.
A. 1 mol/s of methane and 4 mol/s of chlorine
B. 1 mol/s of methane and 1 mol/s of chlorine
Use the shortcut method to account for the effects of temperature.
Figure 14‑9.
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





