If we mix 3 moles of R-134a (1) and 7 moles of R-245fa (2) together at 293
Question:
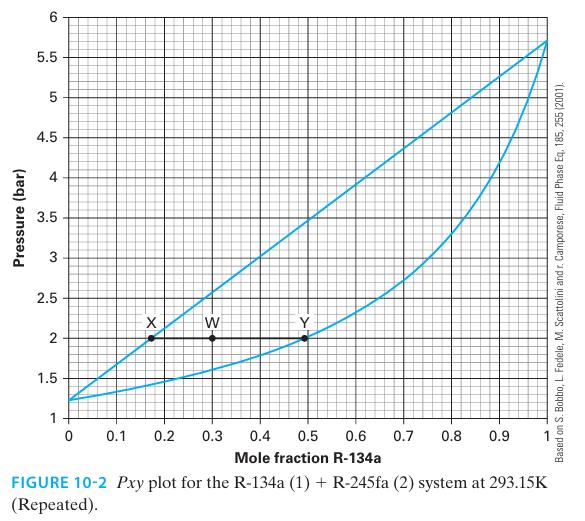
If we mix 3 moles of R-134a (1) and 7 moles of R-245fa (2) together at 293 K and 2.0 bar, what are the composition and amounts of the phase(s) present at equilibrium? Use the lever rule.
Transcribed Image Text:
Pressure (bar) 6 5.5 LO 5 4.5 4 3.5 2.5 2+ 1.5 1 X 0 0.1 0.2 -3- W > 0.3 0.4 0.5 0.6 0.7 0.8 0.9 Mole fraction R-134a FIGURE 10-2 Pxy plot for the R-134a (1) + R-245fa (2) system at 293.15K (Repeated). Based on S. Bobbo, L. Fedele, M. Scattolini and r. Camporese, Fluid Phase Eq. 185, 255 (2001).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
Where is the point on the mixture phase diagram Liquid vapor or both Fortunately we have a phase dia...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Suppose the economy is initially at point A in Exhibit 25-1. If government purchases increase, which point best depicts where the economy will be in the short run as a result of the change in...
-
Kellam Images prints snack bags on long rolls of plastic film. The plant operates 250 days a year. The daily production rate is 6,000 bags, and the daily demand is 3,500 bags sold for $2 each. The...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
(a) Show that (x) = 2x + 3x 36x is not one-to-one on (-, ). (b) Determine the greatest value c such that is one-to-one on (-c, c).
-
Contrast the two types of project life cycles and discuss why it is important to know which type the current project is following.
-
Calculate the following ratios: Return on investment (ROI) Return on capital employed (ROCE) Operating margin Gross margin Sales growth Working capital to sales Gearing Asset turnover
-
What commitment profiles are
-
If Soule Company had net income of $585,000 in 2011 and it experienced a 30% increase in net income over 2010, what was its 2010 net income?
-
Mary is married to Bob (a California Marriage) who is a minority owner of a family owned business (along with his grandparents, parents and two siblings) in closely held Ccorporation which...
-
Does an equimolar mixture of methanol (1) + acetone (2) exist as one or two phases at 101.325 kPa and 334 K? Plot the entire Txy curve to find out. Use Raoults Law to estimate the phase coexistence.
-
What is the entropy change when you mix one mole of butane gas with one mole of propane gas, modeled as an ideal gas mixture? Assume the pressure and temperature of the system are constant during...
-
Solar and lunar eclipses are caused by a fortuitous relationship between the sizes of the Moon and Sun and their distances from Earth. Look up data for the EarthMoon and Earth-Sun distances and the...
-
In this problem you will implement a variant of the List ADT. In particular you will implement the String-List ADT, in a concrete class called SListArray, based on the provided abstract Slist class....
-
Solve (c) 8 WI n=1 5 cos n N5
-
- Pierce Company reported net income of $160,000 with income tax expense of $19,000 for 2020. Depreciation recorded on buildings and equipment amounted to $80,000 for the year. Balances of the...
-
ABC Company had the following results as of 12/31/2020: ABC's hurdle rate is 10% CONTROLLABLE REVENUE CONTROLLABLE COST CONTROLLABLE ASSETS CONTROLLABLE INCOME 21. What is the division's margin? A....
-
A gray kangaroo can bound across a flat stretch of ground with each jump carrying it 10 m from the takeoff point. If the kangaroo leaves the ground at a 20 angle, what are its (a) takeoff speed and...
-
One can show that (see Eyring, Walter, and Kimball, p. 369) where r is the larger of r1 and r2. Substitute this expansion into (9.52). Then multiply the right side by Which from (5.101) equals 1. Use...
-
For each of the following transactions, indicate whether it increases, decreases, or has no effect on the following financial ratios: current ratio, debt-to-equity ratio, profit margin ratio, and...
-
The differential equation model for a certain position control system for a metal cutting tool is where the actual tool position is x; the desired position is x d (t); and K p , K I , and K D are...
-
The differential equation model for a certain speed control system for a vehicle is where the actual speed is , the desired speed is d (t), and K p and K I are constants called the control gains....
-
The differential equation model for the motor torque m(t) required for a certain speed control system is where the desired speed is !d(t) and K is a constant called the control gain. a. Use the...
-
The following items are taken from the financial statements of Grace Company for 2017: $15,000 11,000 28,000 Accounts Payable Accounts Receivable Accumulated Depreciation-Video Equipment Advertising...
-
1 7 8 minutes remaining 2 6 OF 2 6 QUESTIONS REMAINING Question 1 0 3 Points Quark Inc. just began business and made the following four inventory purchases in June: \ table [ [ June 1 , 1 5 0 units,$...
-
#8. Cost Volume Profit Analysis Scenario Mirabel Manufacturing is a small but growing company that manufactures and sells marine sonar equipment. They employee a national sales force and their...

Study smarter with the SolutionInn App


