Maleic anhydride, which has the chemical formula C 2 H 2 (CO) 2 O, can be synthesized
Question:
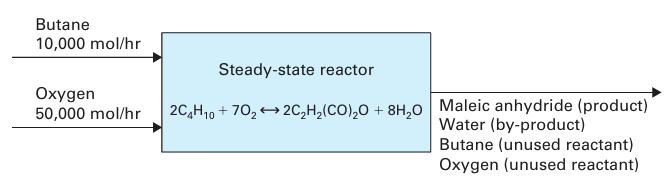
Maleic anhydride, which has the chemical formula C2 H2 (CO)2 O, can be synthesized through the oxidation of n-butane, using the chemical reaction:
![]() 10,000 mol/hr of n-butane and 50,000 mol/hr of oxygen enter a steady-state chemical reactor (Figure 14-3). Derive expressions that quantify the flow rate (in mol/hr) and mole fraction of each reactant and product in the exit stream. The extent of reaction ₤ should be the only unknown in the expressions.
10,000 mol/hr of n-butane and 50,000 mol/hr of oxygen enter a steady-state chemical reactor (Figure 14-3). Derive expressions that quantify the flow rate (in mol/hr) and mole fraction of each reactant and product in the exit stream. The extent of reaction ₤ should be the only unknown in the expressions.
Figure 14‑3.
Transcribed Image Text:
2C₂H₁0 + 70₂2C₂H₂(CO)₂O + 8H₂O 10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
30 mol/s of hydrogen gas and 15 mol/s of air, each compressed to 25 bar, enter a steady state reactor as shown in Figure 15-5, where the nitrogen in the air reacts with the hydrogen to form ammonia:...
-
1. __________ risk involves determining the likelihood that the risk event will occur and the degree of impact the event will have on the project objective. a. Assessing b. Response planning c....
-
What is the difference between Slide Show view and Presenter view in Microsoft PowerPoint?
-
(1 point) Solve each equation for x: (A) Solve 4(x+4) = 8 for x. X = 1 (B) Solve In x + ln(x 4) = 2 for x. X =
-
What conditions must exist to achieve accurate short-run pricing decisions using variable costing?
-
Lisa Winters signed up to take a Latino dance class at the Santa Monica Family YMCA, sort of a dancing-with-the-neighbors experience. However, when she arrived for class on April 17, 2002, the Latino...
-
understand how society at large orients human beings toward continuity in their lives.
-
Judy Olsen, Kristy Johnston, and their mother, Joyce Johnston, owned seventy- eight acres of real property on Eagle Creek in Meagher County, Montana. When Joyce died, she left her interest in the...
-
Hyrkas Corporation's most recent balance sheet and income statement appear below: Statement of Financial Position December 31, Year 2 and Year 1 (in thousands of dollars) Year 2 Year 1 Asset: Current...
-
Arizona has three official courts of record. Judges on the Supreme Court, Court of Appeals, and most Superior Courts are appointed by the Governor. The AZ Constitution requires that all judges must...
-
Ethyl acetate is synthesized from ethanol and acetic acid by the liquid phase esterification reaction: C 2 H 5 OH + CH 3 COOH H 2 O + CH 3 COOC 2 H 5 Ethanol + acetic acid water + ethyl acetate...
-
This sequence of two gas phase reactions can occur in the catalyzed combustion of ammonia: A closed vessel initially contains 5 moles of ammonia and 10 moles of oxygen (Figure 14-2). Assuming these...
-
With reference to Chapter 3 of this book, which covers motivation, attempt to explain why employees at Scania, Angers, are or appear to be well motivated.
-
Alan was rated as excellent on his individual work performance evaluation, earning him $2,000, provided as a merit pay increase. His annual salary this year is $48,000. He works in a team of 3...
-
Josie spends $60 at the end of each month on cigarettes. If shestops smoking and invests the same amount in an investment planpaying 6% compounded monthly, how much will she have after fiveyears? 2...
-
Say we have a Boeing 747 whose longitudinal flight dynamics for a given flight condition may be approximated using the following state equation (uncontrolled motion; thus no need to consider the...
-
a) Provide a brief background of the Honda Motor company and industry, then identify a current ethical issue that has an effect on the industry that the Honda Motor company is operating in. 1b)...
-
If you own, did you consider leasing? If yes, why did you choose a purchase over a lease? If you lease, why did you go with a lease? List the specific advantages you feel you gained by leasing. If...
-
Give three reasons why silicon is the most popular semiconductor used today.
-
Jax Incorporated reports the following data for its only product. The company had no beginning finished goods inventory and it uses absorption costing. $ 57.30 per unit $ 10.30 per unit $ 7.80 per...
-
For the soil profile described in Problem 3.5, estimate an average peak soil friction angle. Use Eq. (3.31b). Problem 3.5 Following is the variation of the field standard penetration number (N 60 )...
-
Repeat Problem 3.7 using Eq. (3.30). Problem 3.7 Following is the variation of the field standard penetration number (N 60 ) in a sand deposit: Depth (m) .............. N 60 1.5...
-
Repeat Problem 3.7 using Eq. (3.29). Use (N 1 ) 60 from Problem 3.5. Problem 3.7 For the soil profile described in Problem 3.5, estimate an average peak soil friction angle. Use Eq. (3.31b). Problem...
-
. In explain the problem Distinguish between a meteor, a meteoroid, a meteorite, an asteroid, and a comet
-
Question 5 of 12 - / 1 View Policies Current Attempt in Progress On January 1, 2020, Larkspur Co. leased a building to Crane Inc. The relevant information related to the lease is as follows. 1. The...
-
detectable before inspection of finished goods. Spoiled units are disposed of at zero net disposal value. Chiplist uses the weighted - average method of process costing. Summary data for September 2...

Study smarter with the SolutionInn App


