Mass diffusion of gases in confined spaces or at very low pressures, called Knudsen diffusion, is quite
Question:
Mass diffusion of gases in confined spaces or at very low pressures, called Knudsen diffusion, is quite different from diffusion at atmospheric pressure. The motion of the gas is governed by collisions of the gas particles with the container walls, not with other molecules. For a container of radius, \(r_{o}\), derive an expression for the self-diffusivity of a gas using kinetic theory and assuming that wall collisions are all-important. Start with equation (3.30) and alter the mean free path. Show that this expression is independent of the gas pressure.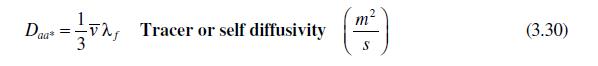
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





