Produce the SLE phase diagram for the p- dichlorobenzene (1) + p-dibromobenzene system at 1 atm. You
Question:
Produce the SLE phase diagram for the p- dichlorobenzene (1) + p-dibromobenzene system at 1 atm. You will do the modeling in two ways and answer each part of the question. Some helpful data are provided in Table P13-30a:
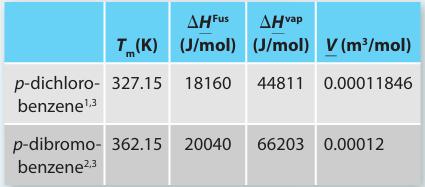
Table P13-30a.
A. Treat the liquid phase as an ideal solution and the solid phase as immiscible. Please plot your phase diagram using the p-dichlorobenzene as the independent variable. What is the eutectic temperature and composition from the model? Compare your work with the experimental data provided in Table P13-30b.
B. Treat the liquid phase as described by regular solution theory using the Scatchard-Hildebrand approach and the solid phase as immiscible. Please plot your phase diagram using the p dichlorobenzene as the independent variable. What is the eutectic temperature and composition from this model? Compare your work with the experimental data provided in Table P13-30b.
C. If you have a liquid mixture that is 76% p- dichlorobenzene and cool it until you meet the liquidus line, what is the composition of the solid precipitate and the temperature at which this occurs for both models? How does this compare to the experimental result?
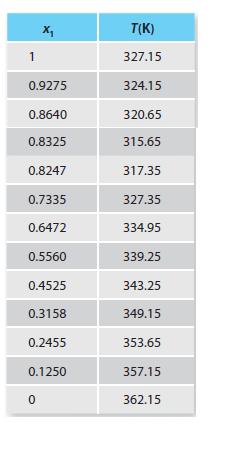
Table P13-30b.
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco




