Prove that the process shown in Figure 4-9 is impossible if the cylinder contains a monatomic ideal
Question:
Prove that the process shown in Figure 4-9 is impossible if the cylinder contains a monatomic ideal gas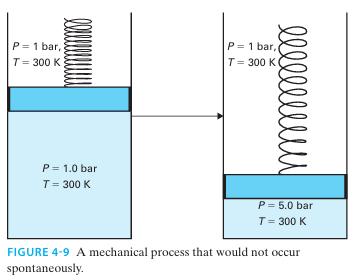
Transcribed Image Text:
P = 1 bar, T= 300 K P = 1.0 bar T = 300 K l l l l l l l l l P= 1 bar, T = 300 K P = 5.0 bar T = 300 K FIGURE 4-9 A mechanical process that would not occur spontaneously.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)

Answered By

Kennedy Odhiambo
As a professional writer, I have been in the field for over 5 years having worked as a lecture in different tertiary institutions across the world. With this impeccable experience, I assure provision of a good and supporting environment for students to learn.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
A monatomic ideal gas follows the cyclic process shown in the figure. The temperature of the point at the bottom left of the triangle is 470.0 K. (a) How much net work does this engine do per cycle?...
-
A monatomic ideal gas at 27°C undergoes a constant pressure process from A to B and a constant volume process from B to C. Find the total work done during these two processes. 1 L 2L V
-
A monatomic ideal gas at 27°C undergoes a constant volume process from A to B and a constant pressure process from B to C. Find the total work done during these two processes. 2 atm B C 1 atm 1L...
-
Tell whether the given side lengths of a triangle can represent a right triangle. 36, 48, and 60
-
Barnes & Noble sells books, magazines, music, and videos through retail stores and on the Web. For a retailer like Barnes & Noble, inventory is a critical element of the business and it is necessary...
-
Shown here are the top 10 U.S. cities ranked by number of hotel rooms sold in a recent year. Suppose four of these cities are selected randomly. a. What is the probability that exactly two cities are...
-
Understand the process of going public. ApendixLO1
-
In a two-player, one-shot simultaneous-move game each player can choose strategy A or strategy B. If both players choose strategy A, each earns a payoff of $500. If both players choose strategy B,...
-
Mush Fitness finished the year with $78,750 in gross receipts and sales. During the year, customers of Mush Fitness were refunded a total of $835. The total compensation expense for their officers...
-
A stream of liquid nitrogen enters an adiabatic, steady-state valve as a saturated liquid at P = 2 MPa. The material leaves the valve at P = 0.6 MPa. Use the data in Figure 2-3 to determine the...
-
A 10 ounce glass of water (half full) is initially at 15C. It is left outside overnight in a location where the air temperature is 5C. By morning the glass is in equilibrium with the surroundings....
-
The trial balance that follows for Shawnee Slopes Company does not balance: Your review of the ledger reveals that each account has a normal balance. You also discover the following errors: 1....
-
What questions would you like to ask of Cassie to better understand any factors that may be affecting Sasha at this time? Growing sunflowers It's now week 6 into the growing sunflowers project. Your...
-
n rope is fixed to a wall and attached to the block such that the rope is parallel to the surface of the wedge. The 12 points) Consider the situation in the figure where a square block (mi) sits...
-
The requirement for extended disclosures for oil and gas reserves described in Chapter 2 followed a Congressional hearing on the poor disclosures that Shell Oil had for its reserves. A.Explain three...
-
Question 9 Big Data techniques implemented in the financial sector include: fraud detection O marketing email campaign O customer relationship management techniques O inventory analysis
-
Problem 8-19A Attaining notfonpmt entity variances The Redmond Management Association held its annual public relations luncheon in April Year 2. Based on the previous year's results, the organization...
-
Which reaction in each of the following pairs will take place more rapidly? a. b. c. d. CH3S Cl CT (CH3)2CHS Cl CI Cl OH + Cl- . H20 OH HCI H20 Cl OHHCI H20 CH2CH2OH (CH3)3CBr
-
Use the information given about the angles and to find the exact value of: (a) sin( + ) (b) cos( + ) (c) sin( - ) (d) tan ( + ) (e) sin(2) (f) cos (2) (g) sin /2 (h) cos/2 cos = 4/5, 0 < < /2; cos =...
-
Grade 1020 steel has a yield strength of 42 ksi and an elastic modulus of 30 Mpsi. Another grade of steel has a yield strength of 132 ksi. What is its elastic modulus?
-
A steel cable of diameter 3/16 in. is attached to an eyebolt and tensioned to 500 lb (Figure P5.4, see on page 226). Calculate the stress in the cable, and express it in the dimensions psi, ksi, Pa,...
-
When a 120-lb woman stands on a snow-covered trail, she sinks slightly into the snow because the compressive stress between her ski boots and the snow is larger than the snow can support without...
-
Wilson Corp. is a wholesaler of imported products. The company had the following opening balances at January 1 , 2 0 2 2 : Accounts receivable $ 2 1 2 , 1 0 0 Allowance for doubtful accounts $ - 2 5...
-
To report the recovery of a casualty loss amount previously deducted on Schedule A, enter the amount on: Form 1040-X, to amend the tax return for the year the casualty loss was deducted. Form 4684,...
-
Winstead Co. invests in $360,000, 6 % , 6-year Birmingham Corp. bonds when the market rate is 8%, Interest is compounded semi-annually. How much cash will Winstead Co, pay for the initial purchase of...

Study smarter with the SolutionInn App


