Reactants A and B combine to form product P in the liquid phase reaction: A 1 B
Question:
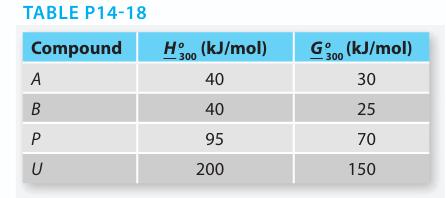
Reactants A and B combine to form product P in the liquid phase reaction: A1 B ↔ P But P reacts further to form an undesired by-product U: 2P ↔ U The Gibbs free energy and enthalpy of each com pound at T = 300 K and P = 1 bar are given in Table P14-18. It is reasonable to assume that both reactions have ΔCP = 0 and that A, B, P, and U form ideal solutions.
The feed entering a steady-state reactor is 1000 mol/hr each of compounds A and B. The reactor is at a uniform pressure of 1 bar.
A. Determine the equilibrium composition of the exit stream at 300 K.
B. Determine the equilibrium composition of the exit stream at 600 K.
C. The second reaction has a larger equilibrium constant than the first, yet there is, at equilibrium, more P than U. Why?
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





