The boiling point of a compound at P = 0.1 MPa is 150 K. The Linde liquefaction
Question:
The boiling point of a compound at P = 0.1 MPa is 150 K. The Linde liquefaction process will be used to produce saturated liquid at P = 0.1 MPa, which has a specific enthalpy of 20 kJ/kg. The Table P5-14 in the right-hand column contains some physical properties of the compound. The steady-state process works as follows. A. Find the flow rate of liquid product leaving the flash chamber.
A. Find the flow rate of liquid product leaving the flash chamber.
B. Find the specific enthalpy (kJ/kg) of the vapor by-product described in step 6.
C. Find the total heat removed by the heat exchangers during step 2, in kJ/min.
D. What is the heat capacity of the compound, in kJ/kg ? K, at ideal gas conditions?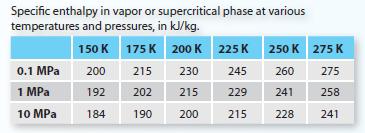
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





