This example revisits the pair of reactions in Example 14-2. 4NH 3 + 5O 2 4NO
Question:
This example revisits the pair of reactions in Example 14-2.
4NH3 + 5O2 ↔ 4NO + 6H2 O
2NO + O2 ↔ 2NO2
100 mol/min of ammonia and 150 mol/min of oxygen enter an isobaric steady state reactor at T = 800 K
A. If the reactions progress to equilibrium at P = 1 bar and T = 800 K, what is the composition of the exiting stream, and at what rate is heat added to or removed from the reactor?
B. Repeat part A for a reactor pressure of 3 bar. Assume ideal gas behavior at this pressure.
Example 14-2.
This sequence of two gas phase reactions can occur in the catalyzed combustion of ammonia:
(R1): 4NH3 + 5O2 ↔4NO + 6H2O
(R2): 2NO + O2 ↔2NO2
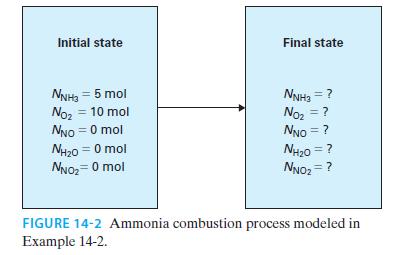
A closed vessel initially contains 5 moles of ammonia and 10 moles of oxygen (Figure 14-2). Assuming these are the only two chemical reactions that occur, write expressions for the mole fractions of each of the species present, in terms of the extents of the two reactions, ₤1 and ₤2.
Step by Step Answer:

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco





