Using data in Appendix C-1, determine the van der Waals parameters a and b for each of
Question:
Using data in Appendix C-1, determine the van der Waals parameters a and b for each of the following compounds.
Appendix C-1
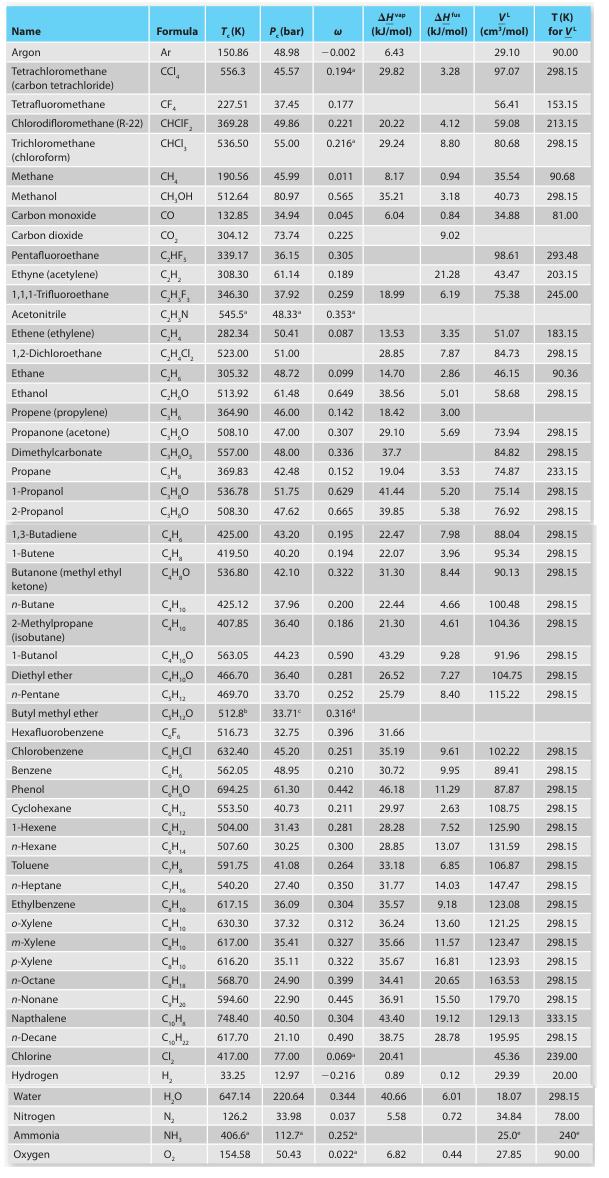
A. Methanol
B. Carbon dioxide
C. Ethanol
D. Butane
E. Octane
Transcribed Image Text:
Name Argon Tetrachloromethane (carbon tetrachloride) Acetonitrile Ethene (ethylene) 1,2-Dichloroethane. Ethane Ethanol Propene (propylene) Propanone (acetone) Dimethylcarbonate Tetrafluoromethane CF 227.51 Chlorodifloromethane (R-22) CHCIF, 369.28 Trichloromethane CHCI, 536.50 (chloroform) Methane Methanol Carbon monoxide Carbon dioxide Pentafluoroethane Ethyne (acetylene) 1,1,1-Trifluoroethane Propane 1-Propanol 2-Propanol 1,3-Butadiene 1-Butene Butanone (methyl ethyl ketone) n-Butane: 2-Methylpropane (isobutane) 1-Butanol Diethyl ether n-Pentane. Butyl methyl ether Hexafluorobenzene Chlorobenzene Benzene. Phenol Cyclohexane 1-Hexene n-Hexane Toluene n-Heptane Ethylbenzene o-Xylene. m-Xylene p-Xylene n-Octane n-Nonanel Napthalene n-Decane Formula Chlorine Hydrogen Water Nitrogen Ammonia Oxygen Ar CCI CH CH,OH CO CO₂ C₂HF, CH₂ C.H.F. C.H.N CH₂ CH.CI, CH C₂H₂O CH CHO C,H,O, CH CHO C₂H₂O CH CH CHO CH₁ CH CHO CH,O C₂H₁₂ C₂H,₂0 CF CH₂C CH CH, CHI CH T. (K) 150.86 556.3 CH₁0 CH CH₂ CH₂ C₁₂H₂z CI₂ H₂ H₂O N₂ NH, 0₂ P. (bar) 48.98 45.57 37.45 49.86 55.00 563.05 44.23 466.70 36.40 469.70 33.70 512.8⁰ 33.71° 516.73 632.40 45.20 562.05 48.95 CHO 694.25 61.30 CH₁₂ 553.50 40.73 CH,₂ 504.00 31.43 CH₁ 507.60 30.25 C₂H₂ CH 425.00 43.20 419.50 40.20 536.80 42.10 425.12 37.96 407.85 36.40 W 190.56 45.99 0.011 8.17 512.64 80.97 0.565 35.21 132.85 34.94 0.045 6.04 304.12 73.74 0.225 339.17 36.15 0.305 308.30 61.14 0.189 346.30 37.92 0.259 545.5" 48.33" 282.34 50.41 523.00 51.00 305.32 48.72 513.92 61.48 364.90 46.00 508.10 47.00 557.00 48.00 369.83 42.48 536.78 51.75 508.30 47.62 591.75 41.08 540.20 27.40 617.15 36.09 630.30 37.32 617.00 35.41 616.20 35.11 568.70 -0.002 0.194 594.60 748.40 617.70 417.00 33.25 647.14 220.64 126.2 33.98 406.6* 112.7 154.58 50.43 0.177 0.221 0.216 0.353" 0.087 0.099 0.649 0.142 0.307 0.336 0.152 0.629 0.665 0.195 0.194 0.322 0.200 0.186 0.590 0.281 0.252 0.3164 AHvap AH fus VL (kJ/mol) (kJ/mol) (cm³/mol) 6.43 29.10 29.82 97.07 20.22 29.24 0.344 0.037 0.252 0.022" 18.99 13.53 28.85 14.70 38.56 18.42 29.10 37.7 19.04 41.44 39.85 22.47 22.07 31.30 32.75 0.396 31.66 0.251 35.19 0.210 30.72 0.442 46.18 0.211 29.97 0.281 28.28 0.300 28.85 0.264 33.18 0.350 31.77 0.304 35.57 0.312 36.24 0.327 35.66 0.322 35.67 24.90 0.399 34.41 22.90 0.445 36.91 40.50 0.304 43.40 21.10 0.490 38.75 77.00 0.069 20.41 12.97 -0.216 0.89 40.66 5.58 22.44 21.30 43.29 26.52 25.79 6.82 3.28 4.12 8.80 0.94 3.18 0.84 9.02 21.28 6.19 3.35 7.87 2.86 5.01 3.00 5.69 3.53 5.20 5.38 7.98 3.96 8.44 4.66 4.61 9.28 7.27 8.40 9.61 9.95 11.29 2.63 7.52 13.07 6.85 14.03 9.18 13.60 11.57 16.81 20.65 15.50 19.12 28.78 0.12 6.01 0.72 0.44 56.41 59.08 80.68 35.54 40.73 34.88 98.61 43.47 75.38 51.07 84.73 46.15 58.68 73.94 84.82 74.87 75.14 76.92 88.04 95.34 90.13 100.48 104.36 T(K) for VL 90.00 298.15 153.15 213.15 298.15 90.68 298.15 81.00 293.48 203.15 245.00 183.15 298.15 90.36 298.15 298.15 298.15 233.15 298.15 298.15 298.15 298.15 298.15 298.15 298.15 91.96 298.15 104.75 298.15 115.22 298.15 102.22 298.15 89.41 298.15 87.87 298.15 108.75 298.15 125.90 298.15 131.59 298.15 106.87 298.15 147.47 298.15 123.08 298.15 121.25 298.15 123.47 298.15 123.93 298.15 163.53 298.15 179.70 298.15 129.13 333.15 195.95 298.15 45.36 239.00 29.39 20.00 18.07 34.84 25.0⁰ 27.85 298.15 78.00 240* 90.00
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:
Students also viewed these Engineering questions
-
Van der Waals Equation and Critical Points (a) In p V- diagrams the slope p/V along an isotherm is never positive. Explain why. (b) Regions where p/V = 0 represent equilibrium between two phases;...
-
Calculate the van der Waals parameters of carbon dioxide from the values of the critical constants and compare your results with the values for and b in Table 7.4. Table 7.4 RedlichKwong van der...
-
The van der Waals equation of state is where a and b are temperature-independent parameters that have different values for each gas. For carbon dioxide, a = 0.3640 Pa m 6 mol 2 and b = 4.267 Ã...
-
solve the following instance of the 0/1 kropsack problem using dynamic programming approach. Assume that the knapsack capacity is 9. Item 1 3 4 weight 6 2 5 7 Benef $8 $4 $6 $10
-
Explain the difference between a standard costing system and the Kaizen costing system popularized in Japan.
-
Refering to the data of Problem 14.24, stored in SpWater , the researchers stated, Some of the benefits of a capable process are increased customer satisfaction, increased operating efficiencies,...
-
17.2A It is widely recognised that budget setting can be mishandled in organisations and may result in some undesirable effects that work against an organisation's best interests. Write a short...
-
The Student Government Association at Middle Carolina University wanted to demonstrate the relationship between the number of beers a student drinks and his or her blood alcohol content (BAC). A...
-
Hooper Retailing Ltd (Hooper) operates a high fashion store in the Sydney CBD. Unfortunately, trading conditions have been difficult as customers are increasingly shopping online and Hooper has...
-
Using data in Appendix C-1, determine the Soave parameters a and b for each of the following compounds at the temperature T = 300 K. Appendix C-1 A. Argon B. Carbon monoxide C. 1-Propanol D. Pentane...
-
Shows a general comparison between arguments a and b, where data_t, the data type of the arguments, is defined (via typedef) to be one of the integer data types listed in Figure 3.1 and either signed...
-
Limestone Corporation is a C corporation that uses the calendar year as its tax year. Its employer identification number is 44-1357913. Limestone is located at 234 Main Street, Anytown, New York...
-
In this problem you will implement a variant of the List ADT. In particular you will implement the String-List ADT, in a concrete class called SListArray, based on the provided abstract Slist class....
-
Solve (c) 8 WI n=1 5 cos n N5
-
- Pierce Company reported net income of $160,000 with income tax expense of $19,000 for 2020. Depreciation recorded on buildings and equipment amounted to $80,000 for the year. Balances of the...
-
ABC Company had the following results as of 12/31/2020: ABC's hurdle rate is 10% CONTROLLABLE REVENUE CONTROLLABLE COST CONTROLLABLE ASSETS CONTROLLABLE INCOME 21. What is the division's margin? A....
-
A gray kangaroo can bound across a flat stretch of ground with each jump carrying it 10 m from the takeoff point. If the kangaroo leaves the ground at a 20 angle, what are its (a) takeoff speed and...
-
A 85.0-kg canoe made of thin aluminum has the shape of half of a hollowed-out log with a radius of 0.475 m and a length of 3.23 m. (a) When this is placed in the water, what percentage of the volume...
-
Imagine a sound wave with a frequency of 1.10 kHz propagating with a speed of 330 m/s. Determine the phase difference in radians between any two points on the wave separated by 10.0 cm.
-
Use the averaging principle developed in Problem 9 to find the temperature distribution of the plate shown in Figure P10, using the 3 3 grid and the given values T a = 150C and T b = 20C. Figure P10...
-
Solve the following equations: 7x + 9y 9z = 22 + 2y 4z = 12 + 5 z %3D -2 Z.
-
The following table shows how many hours in process reactors A and B are required to produce 1 ton each of chemical products 1, 2, and 3. The two reactors are available for 35 and 40 hrs per week,...
-
Carla Willis will invest $33,200 today. She needs $38,488 in 5 years. What annual interest rate must she earn? (Round answer to 0 decimal places, e.g. 7%.)
-
Assume Karen was born January 1 in the year in which the youngest baby boomers were born. Her earnings are $98,000 this year. How much will her social security retirement benefits be if she retires...
-
PLEASE ANSWER ASAP The production department of Zan Corporation has submitted the following forecast of units to be produced by quarter for the upcoming fiscal year: 1st Quarter 2nd Quarter 3rd...

Study smarter with the SolutionInn App


