You are interested in the location of the azeotrope for the acetonitrile (1) + benzene (2) system
Question:
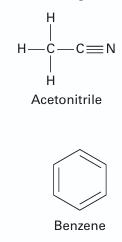
You are interested in the location of the azeotrope for the acetonitrile (1) + benzene (2) system at 346.85 K. However, you only have that information for this system at 318.15 K. At that state (318.15 K) the azeotropic pressure is 37.197 kPa, while the azeotrope is located at x1 = y1 = 0.53 (Palmer and Smith, 1972). Use this information to predict the azeotropic pressure and composition at the temperature of interest (346.85 K). [Note: P at the azeotrope (346.85 K) = 101.325 kPa; x1 = y1 = 0.44 (Lecat, 1946)] Solve the problem using the Peng-Robinson equation of state.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Chemical Engineering Thermodynamics
ISBN: 9781111580704
1st Edition
Authors: Kevin D. Dahm, Donald P. Visco
Question Posted:





