The well-mixed bubbler tank shown in the figure below is used to prepare carbonated water needed for
Question:
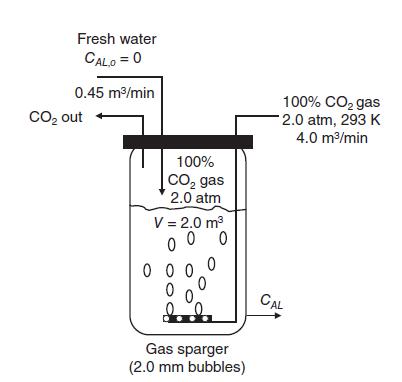
The well-mixed bubbler tank shown in the figure below is used to prepare carbonated water needed for the production of soft drinks. The tank volume is 2.0 m3, and the tank diameter is 1.0 m. The process operates at steady state. Pure water containing no CO2 is continuously added to the tank, and carbonated water containing dissolved CO2 continually exits the tank. Pure carbon dioxide gas at 2.0 atm is bubbled into a tank at a rate of 4.0m3 gas per minute. At these conditions, the “gas holdup” inside the tank is 0.05 m3 gas/m3 liquid, and the average bubble diameter delivered by the fine bubble sparger is 2.0 mm. The CO2 gas not absorbed by the water exits the tank. The process temperature is kept constant at 293 K. The process is liquid film controlling since only pure CO2 is present in the gas phase. The Henry’s law constant for dissolution of CO2 in water is 29.6 atm m3/kgmole at 293 K. The inlet water flow rate of 0.45m3 per minute (25 kgmole H2O/min) contains no dissolved CO2.
a. What is the liquid film mass-transfer coefficient associated with the bubbling of CO2 gas into water?
b. Is the inlet flow rate of CO2 gas sufficient to ensure that the CO2 dissolution is mass-transfer limited?
c. What is the outlet concentration of dissolved CO2?
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781119723547
7th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





