Consider an ideal air-standard Ericsson cycle that has an ideal regenerator, as shown in Fig. P10.44. The
Question:
Consider an ideal air-standard Ericsson cycle that has an ideal regenerator, as shown in Fig. P10.44. The high pressure is 1.5 MPa and the cycle efficiency is 60%. Heat is rejected in the cycle at a temperature of 350 K, and the cycle pressure at the beginning of the isothermal compression process is 150 kPa. Determine the high temperature, the compressor work, and the turbine work per kilogram of air.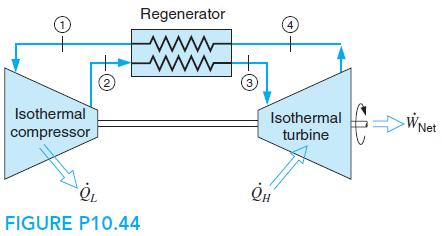
Transcribed Image Text:
Regenerator 1. 4 2) 3 Isothermal Isothermal WNet compressor turbine FIGURE P10.44
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
High temperature 1000K Compressor Work 107 kJkg Turbine Work 162 kJkg The ideal Ericsson cycle consi...View the full answer

Answered By

Firoz K
I have extensive experience in education and tutoring, having worked as a tutor for the past three years in both group and individual settings. During my time as a tutor, I have successfully helped students improve their academic performance in a variety of subjects, including mathematics, science, language arts, and social studies. I have also developed and implemented personalized learning plans and differentiated instruction techniques to accommodate the individual needs of my students. Moreover, I have effectively communicated with parents and teachers to ensure that the students receive the best possible education and guidance. My strong organizational, communication, and problem-solving skills have enabled me to successfully collaborate with students, parents, and teachers in order to provide an effective and enjoyable learning experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Fundamentals Of Thermodynamics
ISBN: 9781118131992
8th Edition
Authors: Claus Borgnakke, Richard E. Sonntag
Question Posted:
Students also viewed these Sciences questions
-
Consider an ideal air-standard Ericsson cycle that has an ideal regenerator as shown in Fig. P11.62, the high pressure is 1 MPa and the cycle efficiency is 70%. Heat is rejected in the cycle at a...
-
Consider a liquid geothermal resource at a temperature of 110oC. An investor is considering a power plant construction on this site, and the investor is asking for your opinion whether this is an...
-
Consider an ideal air standard Brayton cycle in which air into the compressor is at 100 k Pa and 20 o C, and the pressure ratio across the compressor is 12:1 (r p = P2/P1 = 12/1). The maximum...
-
Describe four different definitions of quality.
-
Name three of the four conditions under which automated production lines are appropriate.
-
The wedge is used to level the member. Determine the reversed horizontal force -P that must be applied to pull the wedge out to the left. The coefficient of static friction between the wedge and the...
-
Explain the difference between an unconditional probability and a conditional probability. LO5
-
Janell Arden is a purchasing agentemployee for the A&B Coal Supply partnership. Arden has authority to purchase the coal needed by A&B to satisfy the needs of its customers. While Arden is leaving a...
-
A B G 1 Prepare amortization tables for bonds sold at PAR, a 14 2 3 A Bond sold at PAR 4 5 Face Value $10,000 Note this is a 10% Face Value coupon rate. 6 Interest Rate 10% Interest Rate 7 Bond Term...
-
International Textile Company, Ltd., is a Hong Kong?based firm that distributes textiles worldwide. The company is owned by the Lao family. Should the People's Republic of China continue its economic...
-
An air-standard Ericsson cycle has an ideal regenerator. Heat is supplied at 1000C and heat is rejected at 80C. Pressure at the beginning of the isothermal compression process is 70 kPa. The heat...
-
A gas turbine with air as the working fluid has two ideal turbine sections, as shown in Fig. P10.42, the first of which drives the ideal compressor, with the second producing the power output. The...
-
Using the data in P, draft the journal entries to record: In P, Aero Aluminum Inc. uses a process cost system. The records for May show the following information: 1. The cost of goods received from...
-
Revenue and cash receipts journals; accounts receivable subsidiary and general ledgers Transactions related to revenue and cash receipts completed by Crowne Business Services Co. during the period...
-
Panguitch Company had sales for the year of $100,000. Expenses (except for income taxes) for the year totaled $80,000. Of this $80,000 in expenses, $10,000 is bad debt expense. The tax rules...
-
Assume that the composition of federal outlays and receipts shown in the figure remained the same in 2019. In the figure, the categories "Defense and homeland security" and "Non-defense...
-
1) A car's age is a _ variable. Quantitative O Categorical 2) A car's maker is a variable O Quantitative Categorical 3) A house's square footage is a _________variable O Quantitative O Categorical 4)...
-
The following table shows the distribution of clients by age limits. Use the grouped data formulas to calculate the variance and standard deviation of the ages. Rango de edad Cantidad de clientes...
-
Assume the same facts as in Problem 16. What income, gains, losses, and deductions does Amy report on her income tax return? Based on the information provided, what other calculations is Amy required...
-
Pearl Medavoy will invest $10,240 a year for 20 years in a fund that will earn 10% annual interest. . If the first payment into the fund occurs today, what amount will be in the fund in 20 years? If...
-
Draw a mechanism for each of the following transformations: a. b. c. ,t Dilute H,SO,
-
In this problem, you calculate the error in assuming that ÎH o R is independent of T for the reaction 2CuO(s) 2Cu(s) + O 2 (g). The following data are given at 25°C:
-
If an alkene is protonated and the solvent is an alcohol rather than water, a reaction takes place that is very similar to acid-catalyzed hydration, but in the second step of the mechanism the...
-
Sweeten Company had no jobs in progress at the beginning of March and no beginning inventories. The company has two manufacturing departments --Molding and Fabrication. It started, completed, and...
-
Horizontal Analysis The comparative accounts payable and long-term debt balances of a company are provided below. Current Year Previous Year Accounts payable $47,286 $63,900 Long-term debt 85,492...
-
On January 1, Year 1, Price Company issued $140,000 of five-year, 7 percent bonds at 97. Interest is payable annually on December 31. The discount is amortized using the straight-line method. Record...

Study smarter with the SolutionInn App


