(A) Determine which set of the following quantum numbers (n, , m , m s )...
Question:
(A) Determine which set of the following quantum numbers (n, ℓ, mℓ, ms) is wrong and indicate why:
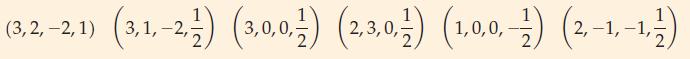
(B) Identify the error in each set of quantum numbers below:
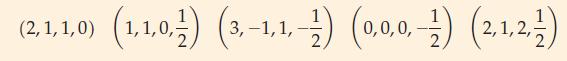
Transcribed Image Text:
(3,2,-21) (3,1,-2) (3,0,0) (230) (1.0.0-) (2-1,-1) (1,0,0,- 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The set of quantum numbers n l ml ms that is wrong is 2 3 0 12 Reasons The azimuthal quantum num...View the full answer

Answered By

Diane Joyce Pastorin
Please accept my enthusiastic application to solutioninn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
IP addressing is located at layer 3 of the OSI model. It is used on all our end devices, our routers, switches, and servers. IP Addressing is critical for allowing communication within our networks...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
Which of the following sets of quantum numbers are not allowed? For each incorrect set, state why it is incorrect. a. n = 3, = 3, m = 0, ms = - 1/2 b. n = 4, = 3, m = 2, ms = - 1/2 c. n = 4, = 1,...
-
The total sales (all credit) of a firm are 6,40,000. It has a gross profit margin of 15 per cent and a current ratio of 2.5. The firm's current liabilities are 96,000; inventories 48,000 and cash...
-
Explain the bottom-of-the-pyramid paradigm. From the multinationals perspective, the BOP a golden opportunity or is it simply a mirage?
-
Suppose that the nine-month LIBOR interest rate is 8% per annum and the six-month LIBOR interest rate is 7.5% per annum (both with actual/365 and continuous compounding). Estimate the three-month...
-
Would you describe Starbucks production/ operations technology in its retail stores as unit, mass, or process? How about in its roasting plants? (Hint: you might need to review material in Chapter...
-
Selected accounts from the chart of accounts of Malone Company are shown below. 101 Cash 201 Accounts Payable 112 Accounts Receivable 401 Sales Revenue 120 Inventory 414 Sales Discounts 126 Supplies...
-
Mr. X will be incorporating his sole proprietorship in the very near future. As such, he is planning on visiting his CPA advisor for tax planning advice. He provided the following list of assets...
-
Background: SO MUCH CANDY DATA, SERIOUSLYCandy hierarchy data for 2017 Boing Boing Halloween candy hierarchy. This is survey data over the span of 4-years. The data is split into 4 separate files....
-
A certain radiation has a wavelength of 574 nm. What is the energy, in joules, of (a) One photon; (b) A mole of photons of this radiation?
-
(A) Write an orbital designation corresponding to the quantum numbers n = 3, = 1, and m = 1. (B) Write all the combinations of quantum numbers that define hydrogen-atom orbitals with the same...
-
Treating ocular discomfort as a nominal scale, assess whether significant differences in ocular discomfort exist between active drug and placebo patients. Report a p-value (two-tailed)? Ophthalmology...
-
For MNEs In light of the pandemic, do you agree that globalisation is in retreat? Why?
-
How do emergent states such as cohesion, potency, and mental models influence team effectiveness and performance in complex and dynamic environments ?
-
2. How do you feel about the progress IKEA Group has made in implementing this plan? I'm looking for analysis for 2-3 pages with a minimum of 3-4 references for this case. Case study: Sustainability...
-
Companies that engage international business do so in pursuit of a broad range of goals. Nonetheless, the text identifies key drivers, noting that the typical company expands operations...
-
How do lifestyle changes, such as urbanization or an aging population, affect consumer needs and preferences in our industry?
-
Find a polynomial function of lowest possible degree whose graph passes through the points (- 2, - 8.2), (- 1, 6.8), (0, 5), (1, - 1), (2, 1.4), and (3, 24.8).
-
Show that every group G with identity e and such that x * x = e for all x G is abelian.
-
Give two reasons why most organizations use an annual period rather than a weekly or monthly period to compute budgeted indirect-cost rates?
-
Distinguish between actual costing and normal costing.
-
Describe two ways in which a house construction company may use job-cost information.
-
Your Corporation uses activity-based costing to determine product costs. The company has provided the following data concerning its activity-based costing system: Activity Cost Pools(and Activity...
-
Maryville Inc. incurred the following costs during August: Raw materials used Direct labor Manufacturing overhead, actual Selling expenses Administrative expenses Interest expense $ 39,300 68,000...
-
A corporation issues a 20 year bond with the final redemption value equal to the face value of $1000, and semiannual coupons of 6.5%. However, the bond is callable at the end of 10 years at $1100,...

Study smarter with the SolutionInn App


