(A) Is the following reaction endothermic or exothermic? (B) Predict whether the following reaction should be exothermic...
Question:
(A) Is the following reaction endothermic or exothermic?
![]()
(B) Predict whether the following reaction should be exothermic or endothermic:
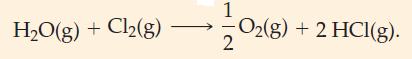
Transcribed Image Text:
CH3COCH3(g) + H₂(g) (CH3)2CH(OH)(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
A The reaction is exothermic This is because the formation of a stable carboncarbon bond and a car...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
For the reaction in Problem 13.69, at 5 bar determine the equilibrium temperature for 95% conversion of coal by this reaction. Is the reaction endothermic or exothermic at this temperature? Problem...
-
The energy profile diagram represents (a) An endothermic reaction (b) An exothermic reaction (c) A fast reaction (d) A termolecular reaction A + B Progress of reaction >
-
Circle the correct answer. (a) The elementary reversible isomerization of A to B was carried out in a packed-bed reactor. The following profiles were obtained: Two trend graph are shown. The...
-
Brothers Willie and Billie each own a gas station for the same national chain. As brothers, they are very competitive. They held a contest to see who could do a better job forecasting sales for their...
-
For each of the following expenditures, indicate the type of account (asset or expense) in which the expenditure should be recorded. Explain your answers. a. $15,000 annual cost of routine repair and...
-
Draw with a class diagram and describe the applicability of the temptation SAP in a scenario, where the government of a country is trying to tempt its citizens to use only the products made within...
-
p. 546Organizations maintain their cultures through attraction, selection, and attrition processes and socialization practices. Organizations change their cultures by changing their leadership or...
-
Joan Whalen has recently been hired as the manager of Jittery Coffee Shop. Jittery Coffee Shop is a national chain of franchised coffee shops. During her first month as store manager, Joan...
-
this is the question unable to figure out?? Brandlin Company of Anaheim, California, purchases materials from a foreign supplier on December 1, 2017 with payment of 17.000 korunas to be made on March...
-
Each of the following ionic compounds consists of a combination of monatomic and polyatomic ions. Represent these compounds with Lewis structures. (a) Al(OH) 3 ; (b) Ca(CN) 2 ; (c) NH 4 F; (d) KClO 3...
-
One of the steps in the formation of monochloromethane (Example 10-15) is the reaction of a gaseous chlorine atom (a chlorine radical) with a molecule of methane. The products are an unstable methyl...
-
What are the advantages of commercial paper in comparison with bank borrowing at the prime rate? What is a disadvantage?
-
4. Write short notes on Wiener Filtering.
-
1.Explain Histogram processing
-
2. Explain Spatial Filtering ?
-
3. Explain the Geometric Transformations used in image restoration. 4.Describe homomorphic filtering
-
5.Explain the different Noise Distribution in detail. UNIT I V 1. What is segmentation? 2. Write the applications of segmentation. 3. What are the three types of discontinuity in digital image? 4....
-
Consider the previous problem, but suppose that D has the value a. 1001000101. b. 1010001111 . c. 0101010101.
-
What can scientists learn by comparing the fossilized skeletons of extinct primates with the bones of modern species?
-
Three grams of musk oil are required for each bottle of Mink Caress, a very popular perfume made by a small company in western Siberia. The cost of the musk oil is 150 roubles per kilogram. (Siberia...
-
The production manager of Rordan Corporation has submitted the following of units to be produced by quarter for the upcoming fiscal year: Each unit requires 0.35 direct labor-hours, and direct...
-
The direct labor budget of Yuvwell Corporation fiscal year contains the following details concerning budgeted direct labor-hours: The companys variable manufacturing overhead rate is $3.25 per direct...
-
Use the future value formula to find the indicated value. n=20; i = 0.03; PMT = $80; FV = ? FV=$1 (Round to the nearest cent.)
-
An unlevered firm has an EBIT = $250,000, aftertax net income = $165,000, and a cost of capital of 12%. A levered firm with the same assets and operations has $1.25 million in face value debt paying...
-
Suppose Mike Inc. has 100 shares outstanding. It receives a constant net income of $1,000 annually and will pay all of it as dividends. What is the stock price today? Assuming the required rate of...

Study smarter with the SolutionInn App


