A portion of the standard electrode potential diagram of selenium is given below. What is the E
Question:
A portion of the standard electrode potential diagram of selenium is given below. What is the E° value for the reduction of H2SeO3 to H2Se in 1 M acid?
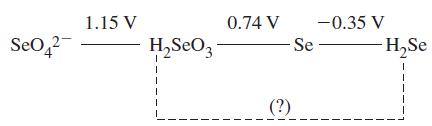
Transcribed Image Text:
Se04² 1.15 V H₂S₂O3 0.74 V -0.35 V -Se- (?) H₂Se 1 1 I T 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To calculate the E value for the reduction of H2SeO3 to H2Se in ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The reaction taking place in an electrochemical cell under standard conditions is Fe 2+ (aq) + Ag + (aq) Fe 3+ (aq) + Ag(s) a. Write two half-equations for this reaction. For each, state whether...
-
The U.S. Department of Energy's Fuel Economy Guide provides fuel efficiency data for cars and trucks. A portion of the data for 311 compact, midsized, and large cars follows. The Class column...
-
The Department of Energy and the U.S. Environmental Protection Agencys 2012 Fuel Economy Guide provides fuel efficiency data for 2012 model year cars and trucks (Department of Energy website, April...
-
Use the data in Exercise 13.28.To familiarize yourself with recursive least squares, estimate the savings functions for 19701981, 19701985, 19701990, and 19701995. Comment on the stability of...
-
Which one of the following is not an example of inventory? a. Cranes at a construction site b. Books on the shelves of a bookstore c. Apples in a supermarket d. Screws to be used in assembling tables...
-
The following is the shareholders' equity section of Suozzi Corp. at December 31, 2017: a The preferred shares have a $2 dividend rate, are cumulative, and participate in distributions in excess of a...
-
Rectify the following errors: (a) A purchase of ` 200 from Ram was omitted to be entered in the purchase book. (b) A credit sale of ` 257 to M/s. Goodluck & Co. was recorded as ` 275. (c) A purchase...
-
Ken, a salaried employee, was terminated from his company in April of this year. Business had been slow since the beginning of the year, and each of the operating plants had laid off workers. Ken's...
-
You are an analyst in a fund analyzing a potential investment. The following assumptions are given: EBITDA is $30 at year 0 and grow 2% each year. The company has $75 million of long-term debt on its...
-
Although relatively rare, all of the following compounds exist. Based on what you know about related compounds (for example, from the periodic table), propose a plausible name or formula for each...
-
Suppose that the sulfur present in seawater as SO 4 2 (2650 mg L 1 ) could be recovered as elemental sulfur. If this sulfur were then converted to H 2 SO 4 , how many cubic kilometers of seawater...
-
Historically, Widgets Manufacturing Inc. produces 250 widgets per day. Recently the new owner bought a new machine to produce more widgets per day. A sample of 16 days production revealed a mean of...
-
Juanita Poblamo makes large ceramic pots for use in outdoor landscape. She currently has two models, one square and the other round. Because of the size of Juanitas creations, only one pot can be...
-
EPI educational products are currently sold without any supplemental materials. The company is considering the inclusion of instructional materials such as an overhead slide presentation, potential...
-
EPI is considering eliminating a product from its ToddleTown Tours collection. This collection is aimed at children one to three years of age and includes tours of a hypothetical town. Two products,...
-
Suppose we estimate the model y i = + u i , where u i N [ 0 , i 2 ] . (a) Show that the OLS estimator of simplifies to ^ = y . (b) Hence directly obtain the variance of y . Show that this...
-
This question presumes access to software that allows NLS and ML estimation. Consider the gamma regression model of Exercise 5-2. An appropriate gamma variate can be generated using \(y=-\lambda \ln...
-
If G is a group, prove that for all a, b G, (a) (a-1)-1 = a (b) (ab)-1 = b-1a-1
-
a) Show that (a, b) := {{a}, {b}} does not satisfy the ordered pair axiom. b) Determine whether each of the following statements is true or false. (Give a reason in each case): (i) {a, b} C (a, b)....
-
Wells Fargo & Company, headquartered in San Francisco, is one of the nations largest financial institutions. It reported the following selected accounts (in millions) as of December 31, 2009....
-
The following stockholders equity accounts, arranged alphabetically, are in the ledger of Patel Corporation at December 31, 2012. Common Stock ($2 stated value) ..................$1,600,000 Paid-in...
-
The following accounts appear in the ledger of Sather Inc. after the books are closed at December 31, 2012. Common Stock (no-par, $1 stated value, 400,000 shares authorized, 250,000 shares issued)...
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


