A saturated aqueous solution of onitrophenol, HOC 6 H 4 NO 2 , has pH = 4.53.
Question:
A saturated aqueous solution of o–nitrophenol, HOC6H4NO2, has pH = 4.53. What is the solubility of o-nitrophenol in water, in grams per liter?
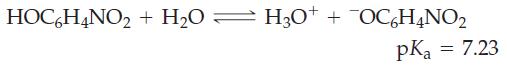
Transcribed Image Text:
HOC6H4NO₂ + H₂O = H3O+ H3O+ + OC6H4NO₂ pKa = 7.23
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To calculate the solubility of onitrophenol in waterwe can use the following equation pH ...View the full answer

Answered By

Sonal Sharma
I have taught chemistry in school as well as college students. I have got the opportunity to deal with their problems in chemistry through interact with them. I am sure once you enjoying the subject you definitely overcome with your subject difficulties .
0.00
0 Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
A saturated aqueous solution of Ca(OH) 2 has a pH of 12.35. What is the solubility ofCa(OH) 2 , expressed in milligrams per 100 mL of solution?
-
A silver rod and a SHE are dipped into a saturated aqueous solution of silver oxalate, Ag2C2O4, at 25C. The measured potential difference between the rod and the SHE is 0.589 V, the rod being...
-
a. Calculate the solubility product of the following solutions: i. A saturated aqueous solution of cadmium sulfide, CdS (solubility = 1.46 10 11 mol dm 3 ) ii. A saturated aqueous solution of...
-
Santa's Helpers Ltd estimates its income taxes at 35% of pre-tax income. For the quarter ended September 30, pre-tax income was $200,000. Prepare the journal entry to record the estimated income...
-
What is leasing? Define, compare, and contrast operating leases and financial (or capital) leases. How does the Financial Accounting Standards Boards Statement No. 13 define a financial (or capital)...
-
An amusement park studied methods for decreasing the waiting time (minutes) for rides by loading and unloading riders more efficiently. Two alternative loading/unloading methods have been proposed....
-
3. MarineCo has no debt but does have unfunded pension liabilities valued at $200 million, recorded as a long-term other liability. MarineCo has detailed in its annual report a potential legal...
-
(a) Your roommate says, Sales taxes are reported as an expense in the income statement. Do you agree? Explain. (b) Pearls Cafe has cash proceeds from sales of $8,550. This amount includes $550 of...
-
prepare a classified balance sheet for the valley pump corporation at decemeber 31, 2021
-
A particular vinegar is found to contain 5.7% acetic acid, CH 3 COOH, by mass. What mass of this vinegar should be diluted with water to produce 0.750 L of a solution with pH = 4.52?
-
What are [H 3 O + ], [OH - ], pH, and pOH of 0.386 M CH 3 NH 2 ?
-
A parallel stream of hydrogen atoms with velocity v = 600 m/s falls normally on a diaphragm with a narrow slit behind which a screen is placed at a distance l = 1.0 m. Using the uncertainty...
-
X Calculate the reaction rate at various conversions, as shown below: FAO -TA -TA 0.2 0.8 Considering that for a PFR: dx V = FAO What is the conversion reached after the 50 m of this PFR?
-
For decades, leaders have talked about flexible working options, yet only few companies were consistently using these practices prior to the global health crisis of 2020. In March 2020, organizations...
-
Manatee Corp. has developed standard costs based on a predicted operating level of 352,000 units of production, which is 80% of capacity. Variable overhead is $281,600 at this level of activity, or...
-
When leaders are facing a crisis or an opportunity, generally, they tend to fall back on the leadership style that has worked for them in the past. Discuss with examples the options that would help...
-
Data Table the pasteet dollar -X Total sales revenues 2 Number of units produced and sold 500,000 units Selling price nt. $ 230,000 te Operating Income Total Investment in assets Variable cost per...
-
Sauer Corp. has a return on assets of 12%. It plans to issue bonds at 8% and use the cash to repurchase stock. What effect will this have on its debt to assets ratio and on its return on common...
-
The following items were displayed in the statement of affairs for Lubbock Company: Fully secured liabilities ......... $90,000 Partially secured liabilities ....... 12,000 Unsecured liabilities...
-
The balance sheet for Bearing Industries Inc. at the end of the current fiscal year indicated the following: Bonds payable, 10% (issued in 2000 due in 2020) $4,000,000 Preferred $5 stock, $100 par...
-
The following information was taken from the financial statements of Finn Resources Inc. for December 31 of the current fiscal year: The net income was $600,000 and the declared dividends on the...
-
The table below shows the stock price, earnings per share, and dividends per share for three companies as of October 2007: (a) Determine the price-earnings ratio and dividend yield for the three...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


