(A) Suppose that the mercury level in Example 6-2 is 7.8 mm higher in the arm open...
Question:
(A) Suppose that the mercury level in Example 6-2 is 7.8 mm higher in the arm open to the atmosphere than in the closed arm. What would be the value of Pgas?
(B) Suppose Pbar. and Pgas are those described in Example 6-2, but the manometer is filled with liquid glycerol (d = 1.26 g/cm3) instead of mercury. What would be the difference in the two levels of the liquid?
Example 6-2
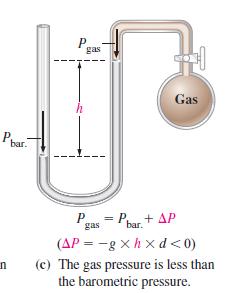
When the manometer in Figure 6-5(c) is filled with liquid mercury (d = 13.6 g/cm3) the barometric pressure is 748.2 mmHg, and the difference in mercury levels is 8.6 mmHg. What is the gas pressure Pgas?
Figure 6-5(c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





