A system containing four neon atoms is confined to a one-dimensional box. The system undergoes an expansion
Question:
A system containing four neon atoms is confined to a one-dimensional box. The system undergoes an expansion from 905 pm to 1810 pm at a fixed total energy of 14.0 x 10-24 J.
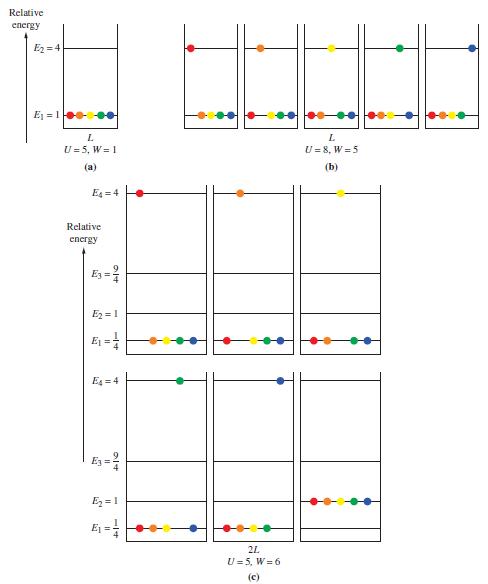
(a) Determine the number of microstates for both the initial and final states of the system by illustrating, in the manner of Figure 13-1, the various possibilities for distributing the atoms among the particle-in-a-box energy levels. Use a different color for each atom.
(b) Calculate the entropy change for the system.
Figure 13-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





