Question: (A) Use data from Tables 19.1 and 23.4 to determine whether nitric acid can be used to oxidize V 3+ (aq) to VO 2+ (aq)
(A) Use data from Tables 19.1 and 23.4 to determine whether nitric acid can be used to oxidize V3+(aq) to VO2+(aq) for standard-state conditions. If so, write a balanced equation for the reaction.
(B) Select a reducing agent from Table 19.1 that can be used to reduce VO2+(aq) to V2+(aq) for standard-state conditions in acidic solution. Consider that the reduction occurs in two stages: VO2+(aq) → V3+(aq) → V2+(aq), but note that the V2+(aq) must not be reduced to V(s).
Tables 19.1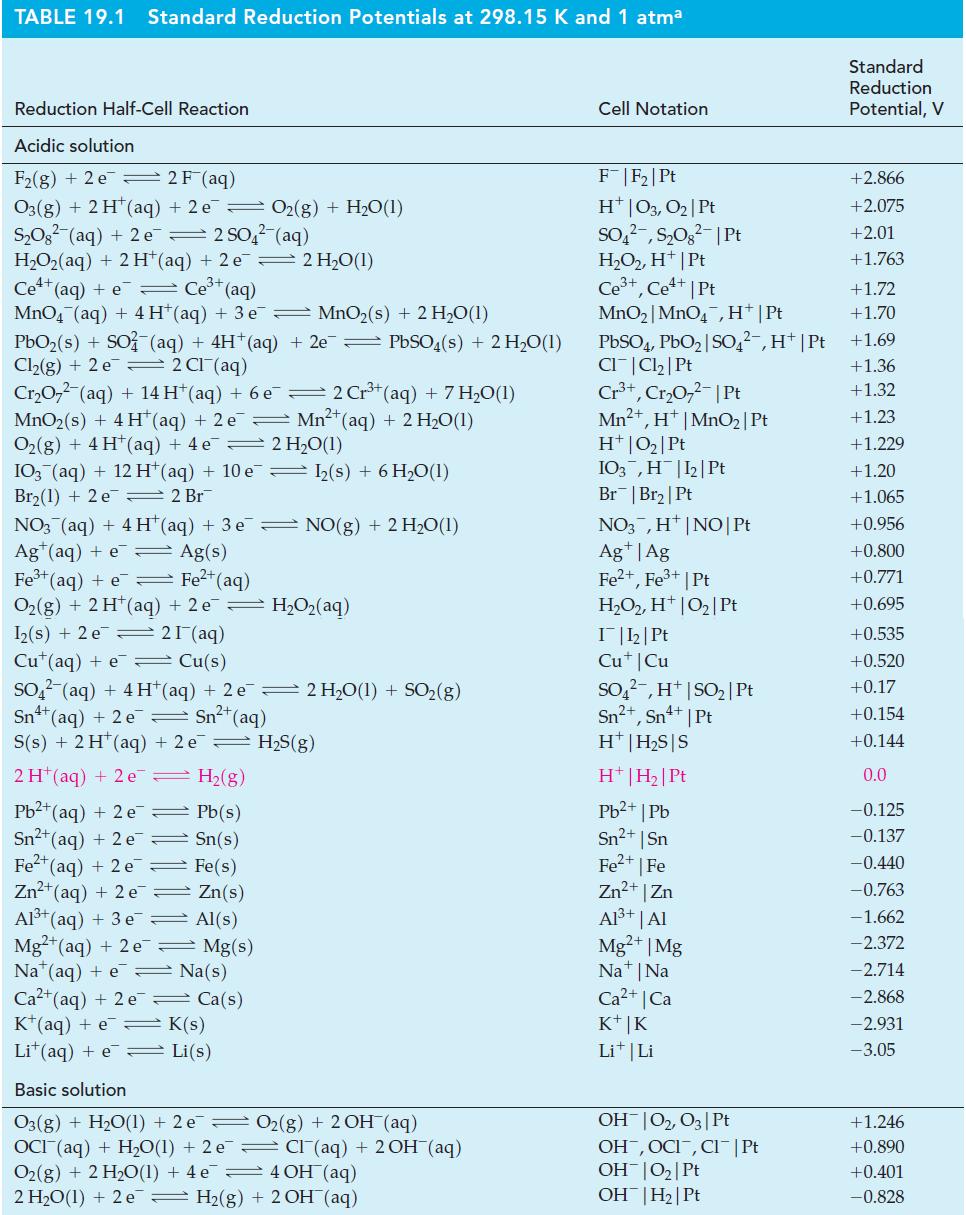
Tables 23.4
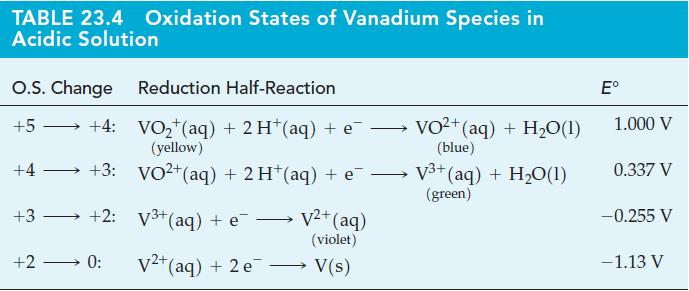
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma Reduction Half-Cell Reaction Acidic solution F(g) + 2 e 2 F (aq) O3(g) + 2 H+ (aq) + 2 e O(g) + HO(1) SO (aq) + 2 e 2 SO4(aq) H,Oz(aq) + 2H*(aq) +2e
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


