(A) What is the value of K for the dissociation of HF(g) into its elements at 298...
Question:
(A) What is the value of K for the dissociation of HF(g) into its elements at 298 K? Use data from Table 22.6.
(B) Use data from Table 22.6 to determine K and the percent dissociation of HCl(g) into its elements at 298 K.
Table 22.6
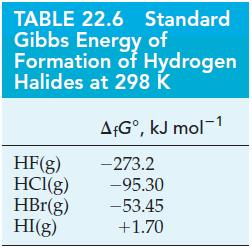
Transcribed Image Text:
TABLE 22.6 Standard Gibbs Energy of Formation of Hydrogen Halides at 298 K AfGº, kJ mol-1 -273.2 HF(g) HCl(g) -95.30 HBr(g) -53.45 HI(g) +1.70
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
A Calculating the value of K for the dissociation of HFg into its elements at 298 K Equation 2HFg H2...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
What is the value of K for the dissociation of HI(g) into its elements at 298 K?
-
(A) Use data from Example 15-2 to determine the value of K at 298 K for the reaction (B) For the reaction NO(g) + 1/2 O 2 (g) NO 2 (g) at 184 C, K = 1.2 x 10 2 . What is the value of K at 184 C for...
-
Simba and Disney, LP (a partnership) each own 1,500 shares in Lion King, Inc. Disney, LP is owned 1/3 by Simba. Simba and Disney have owned the shares in Lion King Inc. for 15 years. Simba, wanting...
-
In a country with a fixed exchange rate system the rise of inflation will result in: O Home currency depreciation Currency appreciation in real terms Floating of home currency O Inflow of foreign...
-
What danger is there in basing a managers bonus on reported net income?
-
On November 30, 2016, there was a fire in the factory of Able Manufacturing Limited, where you work as the controller. The work in process inventory was completely destroyed, but both the materials...
-
If the old forecast is 100 and the latest actual demand is 85, what is the exponentially smoothed forecast for the next period? Alpha is 0.2. LO.1
-
Scores on a marketing exam are known to be normally distributed with mean and standard deviation of 60 and 20, respectively. a. Find the probability that a randomly selected student scores between 50...
-
Small Business Dilemma Long-Term Financing Decision by the Sports Exports Company The Sports Exports Company continues to focus on producing footballs in the United States and exporting them to the...
-
Fluorine is able to stabilize elements in very high oxidation states. For each of the elements Na, Mg, Al, Si, P, S, and Cl, give the formula of the highest oxidationstate of fluoride that is known...
-
Do you expect the ions ICl 2 + and ICl 2 to have the same shape? Explain.
-
Par Corporation and its 100 percent-owned subsidiary, Sam Corporation, are members of an affiliated group with pretax accounting incomes as follows (in thousands): The gain reported by Par relates to...
-
Why do you think it is important to consider only relevant costs when conducting a differential analysis for a major purchase? Why not consider all possible costs in your decision? provide specific...
-
How do power dynamics and influence tactics shape decision-making processes and organizational politics within hierarchical structures ?
-
How do I answer these given the information below? Loan Amount? Loan to Value? Loan to Cost? Payment amount? Loan Balance at Maturity? Given Information: Property Cost: $1,000,000 Bank Policy on LTV:...
-
In your initial post, first do the following: Use scholarly references to define Project Management (PM), Systems Development Life Cycle (SDLC), and Application Life Cycle (AL). Then, in the same...
-
How do concepts of diversity and inclusion vary across different cultural and geographical contexts, and what strategies can multinational organizations employ to navigate these variations...
-
(a) Determine whether each of the following pairs of integers is congruent modulo 8. (i) 62,118 (ii) -43,-237 (iii) -90, 230 (b) Determine whether each of the following pairs of integers is congruent...
-
1. Advertising for eyeglasses _________ (increases/decreases) the price of eyeglasses because advertising promotes _________. 2. An advertisement that succeeds in getting consumers to try the product...
-
Information about Sunburst is presented in E6-4. Additional data regarding the company's sales of Xpert snowboards are provided below. Assume that Sunburst uses a perpetual inventory system....
-
Brooks Hardware reported cost of goods sold as follows. Brooks made two errors:1. 2011 ending inventory was overstated by $2,000.2. 2012 ending inventory was understated by $5,000.InstructionsCompute...
-
Sprague Company reported these income statement data for a 2-year period. Sprague Company uses a periodic inventory system. The inventories at January 1, 2011, and December 31, 2012, are correct....
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


