Ants release formic acid (HCOOH) when they bite. Use the data in Table 7.2 and the standard
Question:
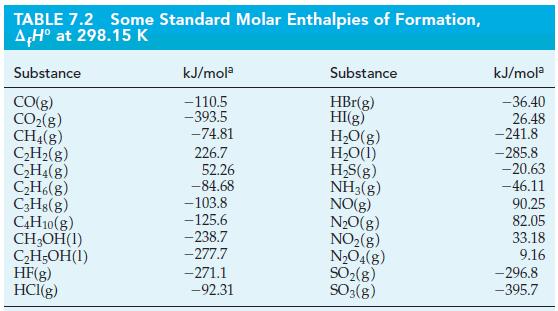
Ants release formic acid (HCOOH) when they bite. Use the data in Table 7.2 and the standard enthalpy of combustion for formic acid (ΔrH° = -255 kJ/mol) to calculate the standard enthalpy of formation for formic acid.
Table 7.2
Transcribed Image Text:
TABLE 7.2 Some Standard Molar Enthalpies of Formation, AH° at 298.15 K Substance CO(g) CO₂(g) CH4(8) C₂H₂(g) C₂H4(8) C₂H6(g) C3H8(g) C4H10(g) CH₂OH(1) C₂H5OH(1) HF(g) HCl(g) kJ/mola -110.5 -393.5 -74.81 226.7 52.26 -84.68 -103.8 -125.6 -238.7 -277.7 -271.1 -92.31 Substance HBr(g) HI(g) H₂O(g) H₂O(1) H₂S(g) NH3(g) NO(g) N₂O(g) NO₂(g) N₂O4(g) SO₂(g) SO3(g) kJ/mola -36.40 26.48 -241.8 -285.8 -20.63 -46.11 90.25 82.05 33.18 9.16 -296.8 -395.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The standard enthalpy change for the combustion of formic acid can be represented as follows HCOOHI ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The standard enthalpy of combustion of ethene gas [C2H4(g)] is 1411.1 kJ/ mol at 298 K. Given the following enthalpies of formation, calculate Hof for C2H4(g). CO2(g) 393.5 kJ/ mol H2O(l) 285.8 kJ/...
-
The standard enthalpy of combustion of cyclopropane is 2091 kJ mol 1 at 25C. From this information and enthalpy of formation data for CO 2 (g) and H 2 O(g), calculate the enthalpy of formation of...
-
The standard enthalpy of combustion of solid urea (CO (NH2)2) is -632 kl mol-1 at 298 K and its standard molar entropy is 104.60 J K-1 mol-1, Calculate the standard Gibbs energy of formation of urea...
-
Consider the following types of images: i. Real, inverted and highly diminished image. ii. Real, inverted and enlarged image. iii. Virtual, erect and enlarged image. iv. Virtual, erect and diminished...
-
Managerial accounting is more than recording, maintaining, and reporting financial results. Managerial accountants must provide managers with both financial and nonfinancial information including...
-
After it is written and time has passed, will an interest rate swap always be equal to zero value? Explain.
-
MIRR A project has the following cash flows: 0 2 3 2$500 $202 2$X $196 $350 $451 1 4 5 This project requires two outflows at Years 0 and 2, but the remaining cash flows are positive. Its WACC is 10%,...
-
You are a public accountant with many small business clients. During a recent visit to a clients business, the bookkeeper approached you with a problem. The columns of the trial balance were not...
-
Tami Tyler opened Tamis Creations, Inc., a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain on Ms....
-
Kevin has met with you and Brenda. Brenda has explained his current duties as an enduring power of attorney holder. She has also provided Kevin with a copy of Norman's will and briefly explained his...
-
Calculate the enthalpy of combustion for lactic acid by using the data in Table 7.2 and the standard enthalpy of formation for lactic acid [CH 3 CH(OH)COOH(s)]: f H = -694.0 kJ/mol. Table 7.2 TABLE...
-
The decomposition of limestone, CaCO 3 (s), into quicklime, CaO(s), and CO 2 (g) is carried out in a gas-fired kiln. Use data from Appendix D to determine how much heat is required to decompose 1.35...
-
The file m-fedip.txt contains year, month, effective federal funds rate, and the industrial production index from July 1954 to December 2003. The industrial production index is seasonally adjusted....
-
Consider a parcel of land that contains an even ages stand of trees currently of age in A in t=0. you have to decide how much longer to allow this stand to grow given that when you cut the stand, you...
-
What does the company report for the following accounts for the most current fiscal year:Enter your answer in thousands.a . Cash$fill in the blank 1 1 , 1 5 4 , 8 6 7 b . Short - term investments (...
-
Consider the translational mechanical system with a nonlinear spring shown below. The spring is defined by s(t)=ks(t), where x(t) is the spring length and f(t) the spring force. Nonlinear spring 0000...
-
Haley Romeros had just been appointed vice president of the Rocky Mountain Region of the Bank Services Corporation (BSC). The company provides check processing services for small banks. The banks...
-
Draw a simple but complete hydraulic circuit diagram to drive two actuators, one of which must be connected to a pressure reducing valve to control its pressure because of the delicacy of the task...
-
Using data from Problems 8 and 10, determine the necessary horsepower for the machine tool to make this cut. Data From Problem 8 Cutting speed .............500 ft/min Feed...........................
-
Heineken N.V., a global brewer based in the Netherlands, reports the following balance sheet accounts for the year ended December 31, 2016 (euros in millions). Prepare the balance sheet for this...
-
Capitalization of Interest on July 31, 2010, Bismarck Company engaged Duval Tooling Company to construct a special-purpose piece of factory machinery. Construction was begun immediately and was...
-
Capitalization of Interest the following three situations involve the capitalization of interest. Situation I On January 1, 2010, Columbia, Inc. signed a fixed-price contract to have Builder...
-
Entries for Equipment Acquisitions Chopin Engineering Corporation purchased conveyor equipment with a list price of $15,000. Presented below are three independent cases related to the equipment....
-
J owns an 80% interest in the capital and profits of the JL partnership. On April 1, 20x1, J bought from the partnership a warehouse that had been used in the business at its FMV of $60,000. The...
-
Can you please do the ratio analysis schedule using Normal formula? i mean without excel because i have to write the steps in details Following are data from the statements of two companies (Tamimi...
-
Expert Q&A Use the following information to answer Questions through 10. A company had the following inventory transactions for the month of April The company had no beginning inventory on hand on...

Study smarter with the SolutionInn App


