At room temperature, iron crystallizes in a bcc structure. By X-ray diffraction, the edge of the cubic
Question:
At room temperature, iron crystallizes in a bcc structure. By X-ray diffraction, the edge of the cubic cell corresponding to Figure 12-45 is found to be 287 pm. What is the radius of an iron atom?
Figure 12-45
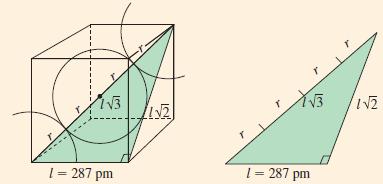
Transcribed Image Text:
13 1 = 287 pm W21 YB 1 = 287 pm IN2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Analyze Nine atoms are associated with a bcc unit cell One atom is located at each of ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use data from Example 12-9, together with the molar mass of Fe and the Avogadro constant, to calculate the density of iron. Example 12-9 At room temperature, iron crystallizes in a bcc structure. By...
-
Metallic iron crystallizes in a cubic lattice. The unit cell edge length is 287 pm. The density of iron is 7.87 g/cm3. How many iron atoms are within a unit cell?
-
At room temperature and pressure RbI crystallizes with the NaCl-type structure. (a) Use ionic radii to predict the length of the cubic unit cell edge. (b) Use this value to estimate the density. (c)...
-
The following trial balance is taken from the General Fund of the City of Jennings for the year ending December 31, 2017. Prepare a condensed statement of revenues, expenditures, and other changes in...
-
Melvin executed and delivered to Dawkins a negotiable promissory note payable to the order of Dawkins as payment for one hundred bushels of wheat Dawkins had sold to Melvin. Dawkins indorsed the note...
-
AP Selected financial data of two competitors, Target and Walmart, are presented here. (All dollars are in millions.) Suppose the data were taken from the 2025 financial statements of each company....
-
Discuss how crisis preparedness contributes to organizational resilience.
-
During late 2003, National Public Radio (NPR) announced a $200 million bequest from the estate of Joan B. Kroc. Mrs. Kroc, widow of McDonalds founder Ray A. Kroc, was a long-time supporter of public...
-
Question 3 3 points Save Anne Use the same information given below for following 3 questions. (Q2-04) Keene, Inc. is considering a new four-year expansion project that requires an initial fixed asset...
-
Use Worksheet 14.1 to help Paul and Crystal Meyer, whod like to retire in about 20 years. Both have promising careers, and both make good money. As a result, theyre willing to put aside whatever is...
-
Water molecules will form small, stable clusters. Draw one possible water cluster by using six water molecules and maximizing the number of hydrogen bonds for each water molecule.
-
A cylinder containing 151 lb Cl 2 has an inside diameter of 10 in. and a height of 45 in. The gas pressure is 100 psi (1 atm = 14.7 psi) at 20 C. Cl 2 melts at -103 C, boils at -35 C, and has its...
-
Explain the stereotypical portrait of workplace aggression?
-
A CM reactor receives influent containing 10.0 mg/L of tracer for 2 h. Then tracer addition is terminated but the flow remains steady. The volume of the reactor is 10 L and the flow rate is 2 L / h....
-
Solve the given system of equations graphically by using a graphing calculator. y=5x x+y2=81 Find the solution with the smaller x-value. x= y= (Type an integer or a decimal rounded to one decimal...
-
I-The market for Sony's Playstation5 game console has changed from 2021 to 2023. With restrictions from the Covid-19 pandemic ending people are finding other entertainment options available such as...
-
3 Below is financial information for December Inc., which manufactures a single product: 4 5 5 7 3 #units produced October Low activity November High activity 7,000 11,000 Cost of goods manufactured...
-
(b) The satellite's booster rockets fire and lift the satellite to a higher circular orbit of radius 2R1. The satellite follows the path shown in the diagram below, moving a total distance S during...
-
What features will be similar in the IR spectra of the following compounds, and how will their IR spectra differ? H2C CHCHCH and CH CH CHCH,CHCH sDh
-
A random sample of 10 houses heated with natural gas in a particular area, is selected, and the amount of gas (in therms) used during the month of January is determined for each house. The resulting...
-
Sustainable Growth and Outside Financing Youve collected the following information about Bad Company, Inc.: Sales = $170,000 Net income = $16,000 Dividends = $11,500 Total debt = $120,000 Total...
-
Sustainable Growth Rate Country Comfort, Inc., had equity of $145,000 at the beginning of the year. At the end of the year, the company had total assets of $270,000. During the year the company sold...
-
Internal Growth Rates Calculate the internal growth rate for the company in the previous problem. Now calculate the internal growth rate using ROA X b for both beginning of period and end of period...
-
For a company with the characteristics below, what would you expect the sustainable growth rate, g, to be? net income/share = $13.6 return on equity = 12.4% payout ratio = 39.9% plowback ratio =...
-
With an initial cost of $100,000, a WACC of 15%, and subsequent cash flows for years 1, 2, 3 of $25,000, $50,000, $75,000, in how many years will break even occur? Use non-discounted cash flows for...
-
Last month, Kaitlin's average daily balance on her credit card was $1,180.81. The annual interest rate on that credit card is 17.52%. The minimum payment on that card is the interest charge ( I...

Study smarter with the SolutionInn App


