Question: Based on the results of Exercise 71, which alkane evolves the greatest amount of heat upon combustion on (a) A per mole basis and (b)
Based on the results of Exercise 71, which alkane evolves the greatest amount of heat upon combustion on
(a) A per mole basis and
(b) A per gram basis? Which is the most desirable alkane from the standpoint of reducing the emission of carbon dioxide to the atmosphere? Explain.
Exercise 71
Use data from Table 7.2 to calculate the standard enthalpies of combustion of the four alkane hydrocarbons listed there.
Table 7.2
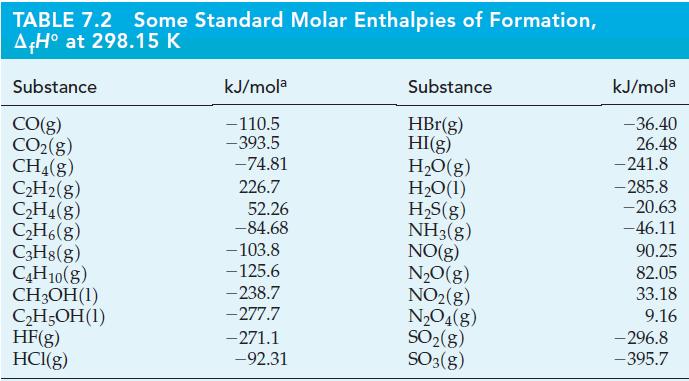
TABLE 7.2 Some Standard Molar Enthalpies of Formation, A+H at 298.15 K Substance CO(g) CO(g) CH4(g) CH(g) CH4(g) CH6(g) C3H8(g) C4H10(g) CHOH(1) CH5OH (1) HF(g) HCl(g) kJ/mola -110.5 -393.5 -74.81 226.7 52.26 -84.68 -103.8 -125.6 -238.7 -277.7 -271.1 -92.31 Substance HBr(g) HI(g) HO(g) HO(1) HS(g) NH3(g) NO(g) NO(g) NO(g) NO4(g) SO(g) SO3(g) kJ/mola -36.40 26.48 -241.8 -285.8 -20.63 -46.11 90.25 82.05 33.18 9.16 -296.8 -395.7
Step by Step Solution
3.59 Rating (152 Votes )
There are 3 Steps involved in it
a Per mole basis To calculate the standard enthalpy of combustion of an alkanewe can use the followi... View full answer

Get step-by-step solutions from verified subject matter experts


