Cinnamaldehyde is the chief constituent of cinnamon oil, which is obtained from the twigs and leaves of
Question:
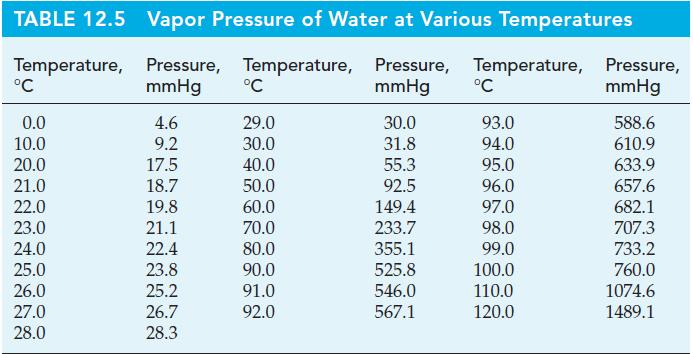
Cinnamaldehyde is the chief constituent of cinnamon oil, which is obtained from the twigs and leaves of cinnamon trees grown in tropical regions. Cinnamon oil is used in the manufacture of food flavorings, perfumes, and cosmetics. The normal boiling point of cinnamaldehyde, C6H5CH=CHCHO, is 246.0 °C, but at this temperature it begins to decompose. As a result, cinnamaldehyde cannot be easily purified by ordinary distillation. A method that can be used instead is steam distillation. A heterogeneous mixture of cinnamaldehyde and water is heated until the sum of the vapor pressures of the two liquids is equal to barometric pressure. At this point, the temperature remains constant as the liquids vaporize. The mixed vapor condenses to produce two immiscible liquids; one liquid is essentially pure water and the other, pure cinnamaldehyde. The following vapor pressures of cinnamaldehyde are given: 1 mmHg at 76.1 °C; 5 mmHg at 105.8 °C; and 10 mmHg at 120.0 °C. Vapor pressures of water are given in Table 12.5.
(a) What is the approximate temperature at which the steam distillation occurs?
(b) The proportions of the two liquids condensed from the vapor is independent of the composition of the boiling mixture, as long as both liquids are present in the boiling mixture. Explain why this is so.
(c) Which of the two liquids, water or cinnamaldehyde, condenses in the greater quantity, by mass? Explain.
Table 12.5
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





