Concerning the rule of thumb stated in Exercise 57, estimate how much faster cooking occurs in a
Question:
Concerning the rule of thumb stated in Exercise 57, estimate how much faster cooking occurs in a pressure cooker with the vapor pressure of water at 2.00 atm instead of in water under normal boiling conditions. Refer to Table 12.5.
Exercise 57
A commonly stated rule of thumb is that reaction rates double for a temperature increase of about 10 °C. (This rule is very often wrong.)
(a) What must be the approximate activation energy for this statement to be true for reactions at about room temperature?
(b) Would you expect this rule of thumb to apply at room temperature for the reaction profiled in Figure 20-10? Explain.
Figure 20-10
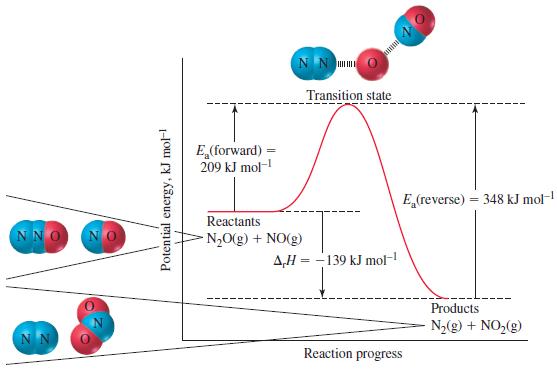
Table 12.5
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





