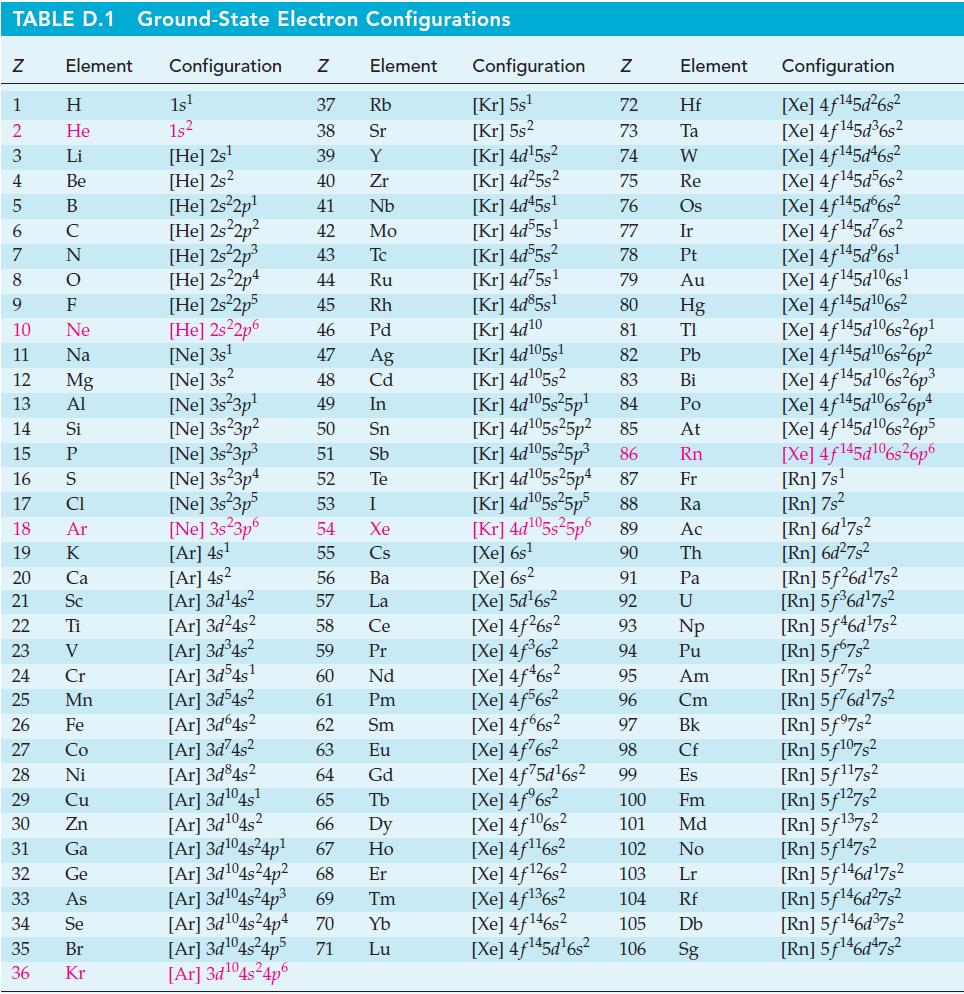
Concerning the thermite reaction, (a) Use data from Appendix D to calculate r H at 298
Question:
Concerning the thermite reaction,
(a) Use data from Appendix D to calculate ΔrH° at 298 K for the reaction below.
![]()
(b) Write an equation for the reaction when MnO2(s) is substituted for Fe2O3(s), and calculate ΔrH° for this reaction.
(c) Show that if MgO were substituted for Fe2O3, the reaction would be endothermic.
Transcribed Image Text:
2 Al(s) + Fe₂O3(s) 2 Fe(s) + Al₂O3(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a Calculate rH at 298 K for the reaction below 2 Als Fe2O3s 2Fes Al2O3s Using data from Appendix D H...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
23+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use data from Appendix C to calculate the equilibrium constant, K, at 298 K for each of the following reactions: H2(g) + 12(g) 2 HI(g) C2H5OH (g)- C2H4(g) + H2O(g)
-
Aqueous tin(II) ion, Sn 2+ (aq), is a good reducing agent. Use data from Appendix D to determine whether Sn 2+ (aq) is a sufficiently good reducing agent to reduce (a) I 2 (s) to I (aq); (b) Fe 3+...
-
Use data from Appendix D to establish for the reaction 2 N 2 O 4 (g) + O 2 (g) 2 N 2 O 5 (g): (a) r G at 298 K for the reaction as written; (b) K at 298 K. TABLE D.1 Ground-State Electron...
-
The need to be liked and to stay on good terms with most other people is the need for? a. Affiliation b. Power c. None of the above d. Achievement
-
Several doctors are considering the purchase of a small real estate business as an investment. Because you have some training in the accounting cycle, they have hired you to review the real estate...
-
Tandy Teck manufactures an electronic component for a high-end computer. The company currently sells 50,000 units a year at a price of $280 per unit. These units are produced using a machine that was...
-
The old deseasonalized forecast is 100 units, and the actual demand for the last month was 150 units. If the seasonal index for the last month is 1.2 and the next month is 0.8, calculate: a. The...
-
You are engaged to examine the financial statements of Horizon Incorporated, which has its own computer installation. During the preliminary understanding phase of your study of Horizons internal...
-
What is the principle of diversification? (500 words limit)
-
Use data from Appendix D (Table D-2) to calculate a value of E for the reduction of Li + (aq) to Li(s), and compare your result with the value listed in Table 21.2. Table D-2 Table 21.2 TABLE D.2...
-
Comment on the feasibility of using a reaction similar to (21.4) to produce (a) Lithium metal from LiCl; (b) Cesium metal from CsCl, with Na(l) as the reducing agent in each case. Consider data from...
-
The enthalpy of vaporization of methanol is 35.27 k] mol-I at its normal boiling point of 64.1oC. Calculate (a) The entropy of vaporization of methanol at this temperature and (b) The entropy change...
-
How do multi-track diplomacy frameworks, integrating official, unofficial, and grassroots efforts at different levels of society, enhance the effectiveness and inclusivity of conflict resolution...
-
As explained by Welch, what should managers do to determine what their own organizations have been up to ?
-
As an administrator how do you demonstrate below situation with suitable examples. 1 Completes tasks to a high standard 2 Demonstrates the necessary level of expertise required to complete tasks and...
-
What influences do the pharmaceutical companies have on psychiatry? What acronym can guide you in formulating a treatment plan (hint: Your instructor emphasizes this when creating a treatment plan,...
-
How do you write a board paper from an article? for example how would y a board paper from the article below look like? Aritcle...
-
Let (S, +, ) and (T, +', ') be two rings. For R = S T, define addition "" and multiplication "" by (s1, t1) (s2, t2) = (S1 + S2, t1 +' t2), (s1 t1) (s2, t2) = (S1 s2, t1. t2). (a) Prove that...
-
You are a U.S. investor who purchased British securities for 2,000 one year ago when the British pound cost U.S. $1.50. What is your total return (based on U.S. dollars) if the value of the...
-
LaTour Inc. is based in France and prepares its financial statements in accordance with IFRS. In 2012, it reported cost of goods sold of 578 million and average inventory of 154 million. Briefly...
-
Franklin Company has the following four items in its ending inventory as of December 31, 2012. The company uses the lower-of-cost-or-net realizable value approach for inventory valuation following...
-
The financial statements of Zetar plc are presented in Appendix C. The companys complete annual report, including the notes to its financial statements. Using the notes to the companys financial...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


