Describe what happens to the following samples in situations like those pictured in Figure 12-31. Be as
Question:
Describe what happens to the following samples in situations like those pictured in Figure 12-31. Be as specific as you can about the temperatures and pressures at which changes occur.
(a) A sample of water is heated from -20 to 200 °C at a constant pressure of 600 Torr.
(b) The pressure on a sample of iodine is increased from 90 mmHg to 100 atm at a constant temperature of 114 °C.
(c) A sample of carbon dioxide at 35 °C is cooled to -100 °C at a constant pressure of 50 atm.
Figure 12-31
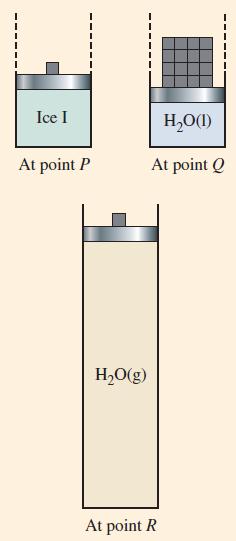
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





