Determine values of K c from the K p values given. (a) SOCl(g) SO(g) + Cl(g) (b)
Question:
Determine values of Kc from the Kp values given.
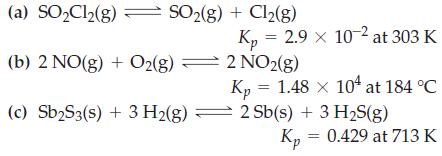
Transcribed Image Text:
(a) SO₂Cl₂(g) SO₂(g) + Cl₂(g) (b) 2 NO(g) + O2(g) (c) Sb₂S3(s) + 3 H₂(g) Kp = 2.9 x 10-2 at 303 K 2 NO2(g) Kp = 1.48 × 104 at 184 °C 2 Sb(s) + 3 H₂S(g) Kp = 0.429 at 713 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
the values of Kc from the Kp values given a SO2Cl2gSO2g C...View the full answer

Answered By

Khurram shahzad
I am an experienced tutor and have more than 7 years’ experience in the field of tutoring. My areas of expertise are Technology, statistics tasks I also tutor in Social Sciences, Humanities, Marketing, Project Management, Geology, Earth Sciences, Life Sciences, Computer Sciences, Physics, Psychology, Law Engineering, Media Studies, IR and many others.
I have been writing blogs, Tech news article, and listicles for American and UK based websites.
4.90+
5+ Reviews
17+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
What is a bureaucrat? Do they operate within a framework that is different from the private sector? If so, explain the differences. What is the effect of the bureaucrat and/or the bureaucracy on the...
-
For the reaction show that Kc = Kp /(RT) Do not use the formula Kp = Kc(RT)n given in the text. See Problem 14.99. COCI2(g) CO(g) + Cl2(g)
-
The equilibrium constant Kc for the reaction is 3.8 Ã 10-5 at 727°C. Calculate Kc and KP for the equilibrium at the same temperature. 12(g)--21(g) 21(g)- 2(g)
-
Who was the petitioner? Who was the respondent? b. In what year was the case heard? c. What tax years did the case address? d. Who was the judge in the case? e. What was the basic issue in the case?...
-
Etsitty Arts, Inc., a leading producer of fine cast silver jewelry, is considering the purchase of new casting equipment that will allow it to expand the product line into award plaques. The proposed...
-
If inventory prices are rising, which inventory costing method should produce the smallest payment for taxes?
-
Choose the final price(s).
-
1. Which of the following statements regarding aggregate planning is true? A) In a pure level strategy, production rates or work force levels are adjusted to match demand requirements over the...
-
You have earned income of $56,000 for last year. You have a defined-contribution plan to which your employer matches your contribution. The employer contributed $2500. How much of an RRSP...
-
After the hypothetical reaction A(g) + B(g) C(g) reaches equilibrium in a closed container, 0.100 mol of the inert gas argon is added. In addition, the volume of the container is decreased....
-
(A) The reaction N 2 O 4 (g) 2 NO 2 (g) is at equilibrium in a 3.00 L cylinder. What would be the effect on the concentrations of N 2 O 4 (g) and NO 2 (g) if the pressure were doubled (that is,...
-
By drinking water after running a marathon, a runner tries to keep his or her body at an optimal level of functioning. This process is called __________.
-
Convex Productions produces full-length motion pictures for distribution worldwide. Convex has just purchased the rights to a movie script entitled Native Sun, which it intends to develop as its next...
-
You are visiting the Engineering Office of Denton Hospital, as part of a consulting project. You notice some charts on one wall which look familiar to you: One of the employees notices you reading...
-
Richmond Clinic has obtained the following estimates for its costs of debt and equity at different capital structures: What is the firms optimal capital structure? (Hint: Calculate its corporate cost...
-
Suppose a sample yields estimates \(\widehat{\theta}_{1}=5, \widehat{\theta}_{2}=3\), se \(\left[\widehat{\theta}_{1} ight]=2\), and se \(\left[\widehat{\theta}_{2} ight]=1\) and the correlation...
-
Helium expands in a nozzle from \(0.8 \mathrm{MPa}, 500 \mathrm{~K}\), and negligible velocity to \(0.1 \mathrm{MPa}\). Calculate the throat and exit areas for a mass flow rate of \(0.34 \mathrm{~kg}...
-
Gary Theater is in the Hoosier Mall. A cashier's booth is located near the entrance to the theater. Two cashiers are employed. One works from 1:00 to 5:00 P.M., the other from 5:00 to 9:00 P.M. Each...
-
The following selected information was taken from Sun Valley Citys general fund statement of revenues, expenditures, and changes in fund balance for the year ended December 31, 2019: Revenues:...
-
Prepare a cost of production report for the Cutting Department of Perma-Wear Carpet Company for October 2010, using the following data and assuming that all materials are added at the beginning of...
-
Performance Castings Inc. casts blades for turbine engines. Within the Casting Department, alloy is first melted in a crucible, then poured into molds to produce the castings. On December 1, there...
-
Franklin Paper Company manufactures newsprint. The product is manufactured in two departments, Papermaking and Converting. Pulp is first placed into a vessel at the beginning of papermaking...
-
Sociology
-
I am unsure how to answer question e as there are two variable changes. In each of the following, you are given two options with selected parameters. In each case, assume the risk-free rate is 6% and...
-
On January 1, Interworks paid a contractor to construct a new cell tower at a cost of $850,000. The tower had an estimated useful life of ten years and a salvage value of $100,000. Interworks...

Study smarter with the SolutionInn App


