Exactly 1.00 mol each of CO and Cl 2 are introduced into an evacuated 1.75 L flask,
Question:
Exactly 1.00 mol each of CO and Cl2 are introduced into an evacuated 1.75 L flask, and the following equilibrium is established at 668 K.
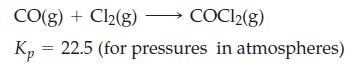
For this equilibrium, calculate
(a) The partial pressure of COCl2(g);
(b) The total gas pressure.
Transcribed Image Text:
CO(g) + Cl₂(g) COC12(g) Kp 22.5 (for pressures in atmospheres)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
The partial pressure of COCl2g and the total gas pressure for the given equilibrium a Partial pressu...View the full answer

Answered By

Muqadas Javed
I am a mentor by profession since seven years. I have been teaching on online forums and in universities. Teaching is my passion therefore i always try to find simple solution for complicated problems or task grasp them so that students can easily grasp them.I will provide you very detailed and self explanatory answers and that will help you to get good grade. I have two slogans: quality solution and on time delivery.
4.60+
24+ Reviews
144+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
QUESTION 1 When propane undergoes complete combustion, the products are carbon dioxide and water.? ? ? ? __ C 3 H 8 (g) + __ O 2 (g) ? __ CO 2 (g) + __ H 2 O(g)What are the respective coefficients...
-
(A) A 5.00 L evacuated flask is filled with 1.86 mol NOBr. At equilibrium at 25 C, there is 0.082 mol of Br 2 present. Determine K c and K p for the reaction 2 NOBr(g) 2 NO(g) + Br 2 (g). (B) 0.100...
-
A mixture of 0.2000 mol of CO2, 0.1000 mol of H2, and 0.1600 mol of H2O is placed in a 2.000-L vessel. The following equilibrium is established at 500 K: (a) Calculate the initial partial pressures...
-
Dr. Bold has a personal automobile policy with liability limits as follows: $100,000/$300,000 BI and $50,000 PD. Dr. Bold is held liable in an accident in which he must pay for bodily injuries as...
-
Data-Check is considering two capital structures. The key information is shown in the following table. Assume a 40% tax rate. a. Calculate two EBIT-EPS coordinates for each of the structures by...
-
(a) Your firm has been hired to build a large government facility near a residential neighbor-hood. A committee of residents has been formed to oppose the building. You have been asked to assist in...
-
6. Why do most young, high-growth companies have negative earnings?
-
Toxaway Telephone Company has a $1,000 par value bond outstanding that pays 6 percent annual interest. If the yield to maturity is 8 percent, and remains so over the remaining life of the bond, the...
-
1.177. B. Mer: 4sin - 3175
-
For the reaction 2 NO 2 (g) 2 NO(g) + O 2 (g), K c = 1.8 x 10 -6 at 184 C. What is the value of K p for this reaction at 184 C, for pressures expressed in atmospheres? (8)702 + NO(g) + O2(g) NO2(g)
-
Concerning the reaction in Exercise 51, if KO 2 (s) and K 2 CO 3 (s) are maintained in contact with air at 1.00 atm and 25 C, in which direction will a net change occur to establish equilibrium?...
-
Harmeling Paint Ball (HPB) Corporation needs a new air compressor that costs $80,000. HPB will need it for only 3 years even though the compressors economic life is long enough so that the lease is...
-
What are the key differences between OLTP (Online Transaction Processing) and OLAP (Online Analytical Processing) databases, and how do they cater to distinct business requirements ?
-
__________ refers to speaking up with good intentions about work-related issues, rather than remaining silent. Multiple Choice Neutralizing Micromanagement Filtering Voice Collaborating
-
Consider Michael Porter's Five Forces Model and use the enclosed form to evaluate the OCSIP industry in Jamaica.
-
Petesy Corporation is preparing its Master Budget for 2019. Budget information is as follows: Sales Production Cost Operating Expenses 2019 1 st Quarter P280,000 P192,000 P64,000 2 nd Quarter 320,000...
-
Design a DFA to recognize any valid fractional numbers of the form . where is at most 3 digits and is any number of digits. However, fractional part can never have more digits than the wholepart. If...
-
Suppose in 2017 that Campbell Soup Company reported average total assets of $6,265 million, net sales of $7,586 million, and net income of $736 million. What was Campbell Soup's return on assets?
-
Respond to the ethical judgments required based on the following scenarios. Scenario 1. Assume you have collected a sample using MUS and that you have evaluated that sample to calculate a total...
-
Rejuvenation Physical Therapy Inc. is planning its cash payments for operations for the third quarter (July?September), 2011. The Accrued Expenses Payable balance on July 1 is $24,000. The budgeted...
-
On January 1, 2010, the controller of Gardeneer Tools Inc. is planning capital expenditures for the years 20102013. The following interviews helped the controller collect the necessary information...
-
Guardian Devices Inc. prepared the following sales budget for the current year: At the end of December 2010, the following unit sales data were reported for the year: For the year ending December 31,...
-
explain the concept of Time Value of Money and provide and example. In addition to your discussion, please explain the differences between Stocks and Bonds
-
Wildhorse Inc. has just paid a dividend of $3.80. An analyst forecasts annual dividend growth of 9 percent for the next five years; then dividends will decrease by 1 percent per year in perpetuity....
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...

Study smarter with the SolutionInn App


