Explain why equation (17.10) fails when applied to dilute solutionsfor example, when you calculate the pH of
Question:
Explain why equation (17.10) fails when applied to dilute solutions—for example, when you calculate the pH of 0.010 M NaH2PO4.
Eq. 17.10
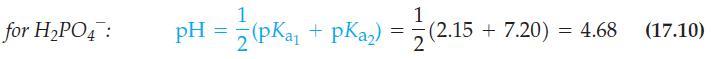
Transcribed Image Text:
for H₂PO4: 1 pH = (pKa₁ + pka₂) = 1 2 (2.15 (2.15 +7.20) = 4.68 (17.10)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Equation 1710 fails when applied to dilute solutions because it does not take into account the activ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Explain why Newtons method fails when applied to the equation 3x = 0 with any initial approximation x 0. Illustrate your explanation with a sketch.
-
Calculate the pH of each of the following solutions (Ka and Kb values are given in Appendix D): (a) 0.095 M propionic acid (C2H5COOH), (b) 0.100 M hydrogen chromate ion (HCrO4-, (c) 0.120 M pyridine...
-
Calculate the pH of a 1 L solution containing (a) 10 mL of 5 M NaOH, (b) 10 mL of 100 mM glycine and 20 mL of 5 M HCl, and (c) 10 mL of 2 M acetic acid and 5 g of sodium acetate (formula weight 82 g ...
-
Revenue for the new startup company "BCB Excavating" for the years 2017 through 2021 have been $543,000, $603,400, $789,000, $845,000, and $889,000 respectively. Year 2017 2018 2019 2020 2021 2022...
-
Mark each of the accounts listed in the following table as follows: a. In column (1), indicate in which statementincome statement (IS) or balance sheet (BS)the account belongs. b. In column (2),...
-
Write a server that tracks the number of the clients connected to the server. When a new connection is established, the count is incremented by 1. The count is stored using a random-access file....
-
What is the anticipated duration of the international assignment?
-
In the blank space beside each numbered balance sheet item, enter the letter of its balance sheet classification. If the item should not appear on the balance sheet, enter a Z in the blank. A....
-
Blank Corporation acquired 100 percent of Faith Corporations common stock on December 31, 20X2, for $214,000. Data from the balance sheets of the two companies included the following amounts as of...
-
A series of titrations of lactic acid, CH 3 CH(OH)COOH (pK a = 3.86) is planned. About 1.00 mmol of the acid will be titrated with NaOH(aq) to a final volume of about 100 mL at the equivalence point....
-
Complete the derivation of equation (17.10) outlined in Are You Wondering 17-1. Then derive equation (17.11). Eq. 17.10 Eq. 17.11 for HPO4: 1 pH = (pKa + pka) = 1 2 (2.15 (2.15 +7.20) = 4.68 (17.10)
-
The following information pertains to First Corporation Ltd: (a) The number of ordinary shares outstanding at the beginning of 20x1 was 30,000,000. (b) On 1 April 20x0, 6,000,000 convertible...
-
On March 6, 2011, Bob's Imports purchased merchandise from Watches Inc. with a list price of \(\$ 31,000\), terms \(2 / 10, n / 45\). On March 10, Bob's returned merchandise to Watches Inc. for...
-
The following events apply to Tops Gift Shop for 2012, its first year of operation: 1. Acquired \(\$ 45,000\) cash from the issue of common stock. 2. Issued common stock to Kayla Taylor, one of the...
-
Indicate whether each of the following costs is a product cost or a period (selling and administrative) cost. a. Transportation-in. b. Insurance on the office building. c. Office supplies. d. Costs...
-
Refer to the information presented in M7-9. Suppose that Juanita has developed a rectangular, medium-size ceramic pot. It requires 3 hours of kiln time; however, two medium-size pots can fit m the...
-
Eclipse Company manufactures a variety of sunglasses. Production information for its most popular line, the Total Eclipse (TE), follows: Suppose that Eclipse has been approached about making a...
-
Find the first and second derivatives of the functions a. b. c. 0. otherwise 0. otherwise 0. otherwise
-
List four items of financial information you consider to be important to a manager of a business that has been operating for a year.
-
Which one(s) of the following are assets traded in financial markets: (a) 6-month Libor (b) A 5-year Treasury bond (c) A FRA contract (d) A caplet (e) Returns on 30-year German Bonds (f) Volatility...
-
Suppose you are given the following information on the spot rate rt: The rt follows: dt =rt + rt dWr. The annual drift is = .01. 15This is the wise because the forward price, Ft], belt belong to the...
-
Suppose at time t = 0, you are given four default-free zero-coupon bond prices P(t, T) with maturities from 1 to 4 years: P(0, 1) = .94, P(0, 2) = .92, P(0, 3) = .87, P(0, 4) .80 (a) How can you fit...
-
How to solve them..equation and explain ..please.. 1. Selected information from the companys financial records is presented below Equipment, December 31, 2013 $300,000 Equipment, December 31, 2014...
-
Your company BMG Inc. has to liquidate some equipment that is being replaced. The originally cost of the equipment is $120,000. The firm has deprecated 65% of the original cost. The salvage value of...
-
Problem 12-22 Net Present Value Analysis [LO12-2] The Sweetwater Candy Company would like to buy a new machine that would automatically "dip" chocolates. The dipping operation currently is done...

Study smarter with the SolutionInn App


